Research Progress of High Temperature Protective Coatings on Molybdenum and Molybdenum Alloy Surfaces
Release time:
2025-01-13
Molybdenum and molybdenum alloys have high melting points, good electrical and thermal conductivity, low coefficients of thermal expansion, excellent corrosion resistance and high-temperature mechanical properties, and are widely used in aerospace, metallurgy, glass, electronics and other industries [1-4]. However, many excellent properties of molybdenum and molybdenum alloys can only be maintained in an inert atmosphere, which is due to their rapid mass loss and rapid reduction of physicochemical properties caused by oxidation in air above 400°C. Thus, the defect of poor resistance to high-temperature oxidation greatly restricts the application of molybdenum and molybdenum alloys in high-temperature oxygen-containing environments [5-6]. Therefore, it is of great significance to study and improve the high-temperature oxidation resistance of molybdenum and molybdenum alloys in order to expand their applications in the field of high-temperature materials.
At present, the ways to improve the resistance of molybdenum and molybdenum alloys to high-temperature oxidation mainly focus on 2 aspects [7-11]: alloying method and surface coating technology. Alloying method is mainly to molybdenum and molybdenum alloys in the addition of alloying elements, this method, although unlike the coating technology needs to be on the surface of the substrate for secondary processing, but also do not have to consider the combination of the coating and the substrate, but molybdenum can be alloyed to a very small extent, adding to improve the oxidation performance of the alloying elements of the more alloying elements, the alloy processing performance deterioration of the performance of high temperature performance (such as high temperature strength, impact resistance, thermal shock resistance and creep resistance, etc.) Therefore, this method has its own limitations [12-13]. It is a very effective method to improve the resistance to high-temperature oxidation of molybdenum and molybdenum alloys through the preparation of high-temperature protective coatings on their surfaces, and the separate design of matrix materials and coatings can not only maintain the mechanical properties of molybdenum and molybdenum alloys at high temperatures, but also improve the resistance to high-temperature oxidation, and thus has a wider range of prospects for application. Researchers at home and abroad have carried out a lot of work around the molybdenum and molybdenum alloy surface high-temperature protective coatings, promoting the development of protective coating material systems and coating preparation technology. This paper summarizes the research results on high temperature protective coatings on the surface of molybdenum and molybdenum alloys at home and abroad, outlines the performance requirements of high temperature protective coatings on the surface of molybdenum and molybdenum alloys, summarizes the system of high temperature protective coatings on the surface of molybdenum and molybdenum alloys, and sums up the application of various coating preparation technologies in the high temperature protective coatings, and finally, the problems existing at this stage of molybdenum and molybdenum alloys and the direction of the future development of the high temperature protective coatings. Prospects.
1 Oxidation behavior of molybdenum
Molybdenum's resistance to high-temperature oxidation is poor, in the air when heated to about 300 ℃ began to oxidize, at this time in the molybdenum surface is covered with a layer of greenish-green oxide film; when heated to about 600 ℃, the formation of a dark green oxide layer; in the temperature range of 600~700 ℃ the formation of volatile oxides MoO3, with the temperature rise, MoO3 volatile formation of white smoke [14], making the quality of molybdenum loss is serious. The oxidation mechanism of molybdenum is different at different oxygen pressures and temperatures. Under constant oxygen pressure at standard atmospheric pressure, molybdenum can be divided into four stages according to its oxidation characteristics at different temperatures [15].
(1) Below 475°C, an adherent oxide film is formed, and the oxidation rate is related to the diffusion rate of metal ions and oxygen through the oxide film, and the oxidation rate in this stage is relatively slow.
(2) At 475~700°C, in addition to the formation of oxide film, accompanied by the volatilization of MoO3, and with the increase in temperature, the volatilization rate is accelerated. The oxidation rate at this stage basically depends on the adsorption, chemical reaction and desorption process on the metal surface.
(3) In 700 ~ 875 ℃, molybdenum on the surface of the oxide film is not generated, can only observe the oxide volatilization, the oxidation rate is completely controlled by the volatilization process, and the oxidation rate at this stage gradually increases.
(4) More than 875 ℃, MoO3 gas in the molybdenum surface of the air over the dense barrier, preventing oxygen and molybdenum surface contact. The oxidation rate is controlled by the diffusion rate of oxygen through the barrier layer. When the temperature rises to 1700 ℃, the oxidation rate is almost unchanged. The oxidation rate at this final stage depends essentially on the surface area of the specimen and the rate of oxygen flow.
2 Performance requirements for high-temperature protective coatings on molybdenum and molybdenum alloy surfaces
The antioxidant performance of the coating is closely related to its chemical composition, microstructure and oxidizing environment. The key to the design of high-temperature protective coatings on the surface of molybdenum and molybdenum alloys is that the coatings can act as an oxygen barrier to isolate the oxidizable substrate from the external oxygen, and must also take into account the stability of the coating organization and structure, the coating defects, the strength of the coating and the substrate interfacial bonding, interfacial physical and chemical compatibility, and oxygen diffusion and other aspects of the requirements. Generally speaking, the high temperature antioxidant coating should have the following characteristics [16].
(1) The coating should have excellent antioxidant properties. Under the high temperature environment, the surface of the coating can form a continuous and dense inert oxide film.
(2) The coating should be dense and complete, with a low oxygen diffusion rate, which can effectively inhibit the diffusion of oxygen to the interior. At the same time, it should also have a good organizational stability, in service is not easy due to the diffusion reaction between the coating or the coating and the substrate within the phase change, which leads to structural and performance degradation.
(3) The thermal expansion coefficient of the coating and the substrate should be matched, while the interface between the coating and the substrate with high bonding strength, good chemical compatibility and mechanical compatibility, so as not to cause the coating cracking due to thermal stresses in the course of use, or even peeling.
(4) Defects such as holes and microcracks inside the coating will affect its antioxidant performance, so the preparation quality of the coating should be ensured to avoid or reduce the number of internal defects as much as possible. At the same time, the preparation process of the coating should be as simple as possible, and low cost, suitable for batch production.
3 molybdenum and molybdenum alloy surface high temperature protective coating system
According to the molybdenum and molybdenum alloy surface high temperature protective coating performance requirements, at this stage applied to the molybdenum and molybdenum alloy surface high temperature protective coatings mainly include silicide coatings, aluminum oxide coatings, heat-resistant alloy coatings, oxide coatings, etc., which to silicide coatings and their modified coatings mainly.
3.1 Silicide coating
MoSi2 as a representative of the silicide coating has a high melting point, moderate density, good thermal stability and other properties, and in the high temperature environment can be oxidized to form a self-healing function and low oxygen permeability of the glassy SiO2 protective film, showing excellent high-temperature antioxidant capacity, and thus become an ideal coating material for molybdenum and molybdenum alloys for high temperature protection [17-18]. physical properties are shown in Table 1.
3.1.1 Single silicide coatings
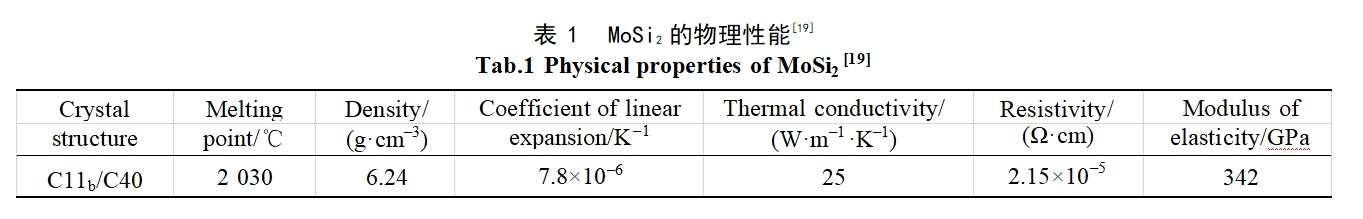
The single MoSi2 coating has the advantages of simple preparation process and low production cost.Zhang et al[20] used liquid-phase silicone penetration technology to prepare Mo-MoSi2 functional gradient coating on the surface of molybdenum substrate, which is dense and smooth without porosity and crack defects, and has good metallurgical bonding with the molybdenum substrate.The Mo-MoSi2 functional gradient coating presents a three-layered structure from the outside to the inside, with an external layer of Si/Mo atomic ratio between 6:1 and 2:1, composed of MoSi2 and Si; an intermediate layer with Si/Mo atomic ratio of about 2:1, composed of MoSi2 and Si; a middle layer with Si/Mo atomic ratio of about 2:1, composed of MoSi2 and MoSi. Mo atomic ratio between 6:1 and 2:1, composed of MoSi2 and Si; the Si/Mo atomic ratio of the middle layer is about 2:1, composed of MoSi2; and the Si/Mo atomic ratio of the transition layer is between 2:1 and 0:1, composed of Mo5Si3 and Mo. They also prepared Si-MoSi2 functional gradient coatings on the surface of molybdenum substrate by liquid-phase silicon infiltration method, which consisted of a Si-MoSi2 layer (2.5 µm), a MoSi2 layer (18 µm), and a Mo-Mo5Si3-Mo3Si layer (2 to 4 µm) [21]. The infiltrated silicon coating has a dense layered structure without microcracks and holes. Silicon is mainly enriched on the coating surface with a maximum mass fraction of about 50%, which inhibits the formation of Mo5Si3 and volatile MoO3 and improves the high temperature oxidation resistance of the coating. The mass gain of the Si-MoSi2 coating was only 0.17% after oxidation at 1600 °C for 70 h. Cai et al [22] prepared Si-Mo coatings on the surface of TZM alloy substrate by slurry sintering method and compared the oxidation properties of the substrate and the coatings at a high temperature of 1650 °C, which consisted of an outer MoSi2 layer and an inner Mo5Si3 interfacial diffusion layer.TZM alloy specimens were oxidized at 1650 °C for about 35 min and then completely ablated, while the Si-Mo coated specimens remained intact after 14 h of oxidation with a mass increment of only 0.816 mg/cm2.
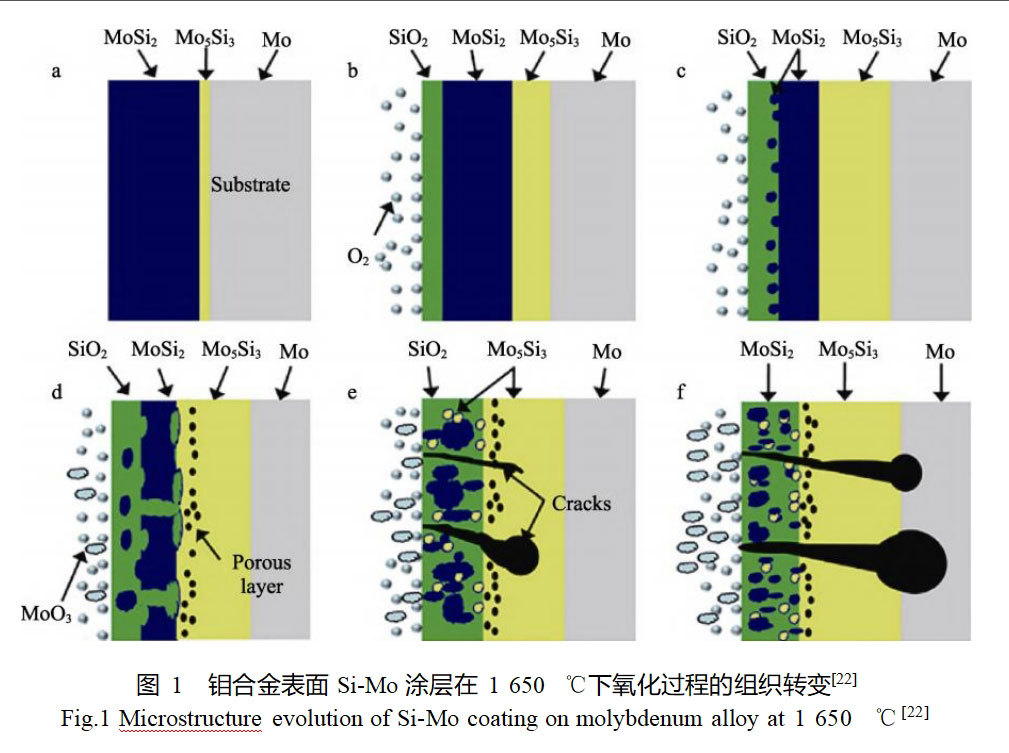
The evolution of high temperature oxidation of Si-Mo coating at 1650°C is shown in Fig. 1. With the prolongation of oxidation time, the surface SiO2 oxide film layer and the Mo5Si3 layer at the interface gradually become thicker, while the MoSi2 layer gradually becomes thinner due to simultaneous depletion by oxidation and interfacial diffusion reaction. When the MoSi2 in the coating is no longer continuously distributed, oxygen enters the coating and reacts with Mo5Si3 and the molybdenum alloy matrix to generate MoO3, and the volatilization of MoO3 leads to the formation of holes and cracks on the coating surface and inside the coating, which ultimately leads to the failure of the coating.Pu et al. [23] firstly prepared a pure molybdenum layer on the surface of the TZM alloy by slurry vacuum sintering, and then used the halide activated embedded infiltration method, which was applied to the deposited pure molybdenum layer. The pure molybdenum layer was firstly prepared on the surface of TZM alloy by slurry vacuum sintering method, followed by halide activated embedded infiltration method. Two types of molybdenum powders with different particle sizes were used as raw materials for the preparation of the pure molybdenum layer, so that two types of MoSi2 coatings were finally obtained by the two-step method. The results show that both coatings have a bilayer structure, with a porous MoSi2 layer on the outside and a dense MoSi2 layer on the inside. By comparing the oxidation performance of the two coatings at 1700°C, it is found that the MoSi2 double-layer coating prepared by fine-grained molybdenum powder has better antioxidant performance and can protect the substrate for up to 6 h. The oxidized coatings show a multilayer structure, with a SiO2 layer, a porous MoSi2 layer, a dense MoSi2 layer, and a Mo5Si3 layer, respectively, from the outside to the inside, as shown in Fig. 2. During the oxidation process, MoSi2 gradually transforms to Mo5Si3, which ultimately leads to the failure of the coating due to silicon depletion.
However, the use of single-component MoSi2 as a protective coating for molybdenum and molybdenum alloy surfaces against oxidation has certain limitations. At low temperatures (400~600°C), the significant volume changes caused by the oxidized MoO3 and SiO2 can easily lead to the catastrophic “pest” phenomenon of MoSi2 [24]. In the mid-temperature stage (600~1000°C), the formation of the complete SiO2 protective film is still inhibited, and SiO2 is more viscous and less mobile below 1000°C. The formation of the SiO2 protective film is also inhibited in the mid-temperature stage (600~1000°C), and the formation of the complete SiO2 protective film is still inhibited. At higher than 1600°C, SiO2 is volatile due to the increase of vapor pressure, which also leads to the failure of the coating, so the antioxidant ability of MoSi2 is limited in the medium-low temperature and ultra-high temperature environments. At the same time, the difference in thermal expansion coefficient between MoSi2 coating and molybdenum substrate leads to poor thermal shock resistance of the coating, and cracks are easily formed inside the coating due to the release of thermal stresses during the cooling process, which seriously affects the integrity of the coating and its ability to protect the substrate. In addition, the solid-phase diffusion between Mo, Si and other elements at high temperatures transforms MoSi2 into Mo5Si3, and this low-silicon Mo5Si3 phase is unable to form a continuous SiO2 protective film during the oxidation process, which results in a rapid decline in the antioxidant performance of the coating. Therefore, to address the limitations of single MoSi2 coatings, researchers have focused on both coating composition and structural design to improve the antioxidant performance by preparing modified silicide coatings.
3.1.2 Modified silicide coatings
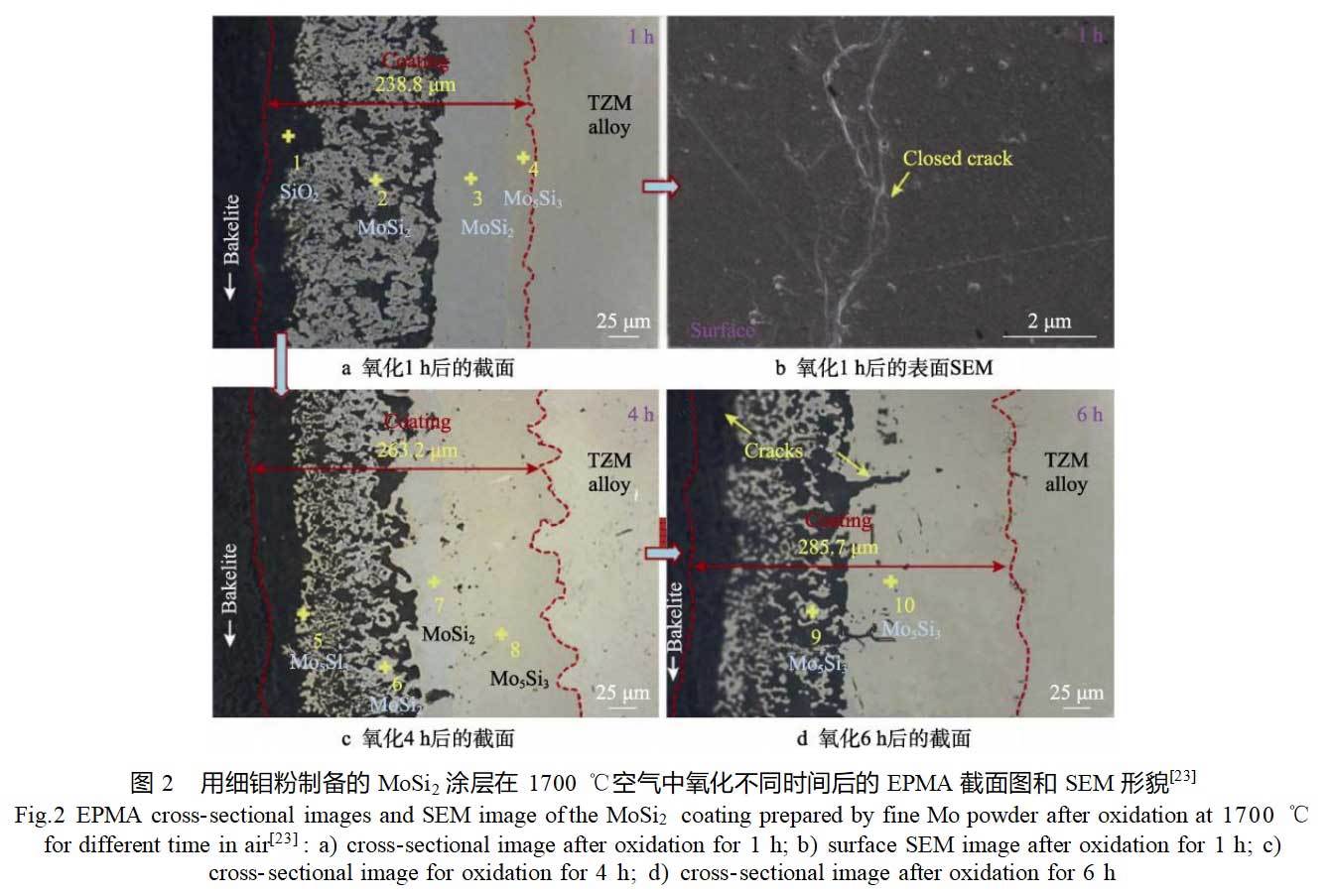
The coating composition design is mainly based on the introduction of active elements such as B, Al, Ti, or reinforcing phases such as YSZ and Si3N4 to the MoSi2 coating, and modified silicide coatings are obtained through the adjustment of the coating composition [25-27].The formation of low-viscosity B2O3 phase after the oxidation of B elements can increase the mobility of SiO2 and improve its ability to repair the defects of the coating [28].The MoB or Mo5SiB2 phase can also effectively inhibit the diffusion of Si into the substrate, thus the preparation and oxidation properties of B-modified MoSi2 coatings have been the research hotspot of antioxidant coatings on molybdenum surfaces [29-30].Tian et al [29] used the embedded Si-B co-diffusion method to deposit the B-modified MoSi2 coatings on molybdenum substrate surfaces, and the coatings were composed of a MoSi2 layer from the outside and a MoB dispersion layer distributed in the lower region of the MoSi2 layer from the outside to the inside. MoB dispersed phase in its lower region, Mo5Si3 layer, MoB layer and Mo2B inner layer. The variation of B content in the infiltrant (16Si-xB-4NaF-Bal.Al2O3, x=0.5~4), the co-infiltration temperature (1100~1400°C) and the time (2.5~10h) had little effect on the coating structure. The growth of the coatings was mainly dominated by the internal diffusion of Si and B atoms, with Si mainly involved in the formation of MoSi2 and B mainly reacting with MoSi2 to produce the MoB phase and participating in the formation of MoB and Mo2B layers. They [30] also compared the high-temperature oxidation behavior of single MoSi2 coatings and B-modified MoSi2 coatings at different temperatures (600, 1250, and 1500°C.) After 100 h of oxidation at 600°C, the two coatings did not undergo “pest” oxidation. At the early stage of oxidation at 1250 and 1500°C, both coatings lost quality due to the generation of volatile MoO3. After 20 h of oxidation, a complete oxide film was formed on the surface and the mass increased. At 1250°C, the generation of low-viscosity B2O3 on the surface of the B-modified MoSi2 coating promotes the formation of a full-coverage oxide film and reduces the volatilization of MoO3, resulting in a lower oxidation rate for the B-modified MoSi2 coating compared to the single MoSi2 coating. At 1500 °C, this effect is negligible because SiO2 itself has good mobility at high temperatures. It was also found that the MoB layer in the B-modified MoSi2 coating can retard the internal diffusion of Si into the substrate via the MoSi2 layer, and therefore can effectively improve the antioxidant life of the coating.Tian et al [31] investigated the effect of the active element Y on the Si-B composite coatings on the surface of the molybdenum substrate on the basis of the encapsulation of the Si-B co-deposition. They prepared Si-B-Y2O3 and Si-B-Y composite coatings on the surface of molybdenum matrix by using 16Si-4B-xY2O3/Y-4NaF-(76-x)Al2O3 (x=0,0.5,1,2,4,8) as a percolating agent after a 5-h embedding and percolating treatment at 1300 °C. The results show that the Si-B-Y2O3 and Si-B-Y composite coatings have the same structure as the Si-B composite coating. The difference is that the thickness of the two composite coatings is larger than that of the Si-B coating due to the introduction of Y. Y is mainly distributed in the MoSi2 layer, and its content in the Si-B-Y coating is higher than that in the Si-B-Y2O3. The cyclic oxidation performance of the Si-B-Y composite coating is better than that of the Si-B coating at 1000 ℃, which may be attributed to the fact that Y preferentially combines with oxygen in the early stage of oxidation, and thus can inhibit the oxidation of Mo. This may be due to the fact that Y will preferentially combine with oxygen at the early stage of oxidation, thus inhibiting the oxidation of Mo. Zhai et al [25] prepared a MoSi-MoB bilayer coating on the surface of molybdenum substrate using a two-step embedding and infiltration method, and comparatively investigated the high-temperature oxidization performance of the coating and the substrate at 1300℃. The results of high-temperature oxidation experiments showed that the mass loss of the MoSi-MoB bilayer coating was 6.3824 mg/cm2 after oxidation at 1300°C for 15 h, while the mass loss of the molybdenum substrate reached 606.775 mg/cm2 after oxidation for 90 min.
The excellent antioxidant performance of MoSi2-MoB bilayer coating is closely related to the SiO2 oxide film and diffusion barrier layer formed on its surface.The SiO2 layer formed by the oxidation of MoSi2 is continuous and dense and has a low oxygen permeability, so it can effectively retard the internal diffusion of oxygen, as shown in Figure 3. At the same time, the diffusion reaction between the MoSi2 layer and the MoB layer during the oxidation process led to the generation of a Mo5SiB2 diffusion barrier layer at the interface, which is also capable of hindering the internal diffusion of Si into the substrate.Deng et al [32] prepared a multiphase Mo-Si-B ceramic coating on the surface of a molybdenum substrate by using the plasma transfer arc surface treatment technique. The microstructure of the coatings consisted of rod-like Mo5SiB2 dendrites, layered Mo3Si/Mo5SiB2 biphasic eutectic and cellular Mo5Si3 dendrites. The mass loss of molybdenum substrate after oxidation in air at 1300°C for 10 min has reached 141.2 mg/cm2, while the mass loss of Mo-Si-B coating after oxidation for 30 h is only 8.2 mg/cm2, which significantly improves the antioxidant performance of the substrate.After oxidation of the Mo-Si-B coating, a layer of continuous dense borosilicate protective film can be formed on the surface, which can act as an oxygen diffusion blocking layer. It was also found that the antioxidant properties of the Mo5SiB2 dendritic phase in the coating organization were better than those of the Mo3Si/Mo5SiB2 eutectic phase.
Al is a commonly used alloying element to improve the oxidation resistance of materials, which can improve the “pest” oxidation problem of MoSi2 coatings in the low and medium temperature domains, and also help to improve the resistance of the coatings to high temperature oxidation [26,33].Sharifitabar et al. [34] used a commercial Al-12% Si alloy melt as the raw material, and utilized the hot-rolled Al5SiB2 eutectic phase as the raw material to improve the oxidation resistance of the coating. alloy melt as raw material and prepared Mo(Si,Al)2 coatings on molybdenum surfaces using hot-dip plating method, and discussed the effects of dip plating time and temperature. The results show that a uniform Mo(Si,Al)2 coating with a thickness of 40-60 μm can be obtained on the molybdenum surface after 60 min of immersion plating in the melt at 850 ℃; after 60 min of immersion plating in the melt at 950 ℃, a coating with a bilayered structure can be obtained, with the inner layer consisting of nano-Mo5Si3 particulate phases dispersed in an Al8Mo3 matrix and the outer layer consisting of Al8Mo3 and Mo(Si, Al)2 phase; whereas at lower dip coating temperatures (e.g., 750 °C), a longer time is required to obtain the Mo(Si,Al)2 coating.
Figure 3
Majumdar et al [26] prepared MoSi2 coatings and Mo(Si,Al)2 coatings on the surface of TZM alloys by the encapsulated infiltration method and comparatively investigated the high temperature oxidation process of the TZM substrate and the two coatings using thermogravimetric analysis. During the continuous oxidation process, the TZM alloy undergoes mass loss due to the volatilization of the nonmetric molybdenum oxide MoOz (2<z<3) from 200 to 550°C; turns to mass gain due to the formation of MoO3 between the temperatures of 550 and 925°C; and undergoes a rapid mass loss due to the accelerated volatilization of MoO3 when the temperature is higher than 925°C. Compared with TZM alloys, the mass changes of MoSi2 and Mo(Si,Al)2 coatings were very small.After oxidation of the two coatings, SiO2 and Al2O3 oxide films were formed on the surface, respectively, and the growth rate of Al2O3 was slower than that of SiO2.The appearance of small cracks on the surface of the SiO2 oxide film and the distribution of MoO3 whiskers around the SiO2 particles suggested that the “cracks” of MoSi2 coating occurred in the mid-temperature stage. Lange et al. [33] used a combination of magnetron sputtering and subsequent annealing on the surface of Mo- 9Si-8B alloy by magnetron sputtering and subsequent annealing, and the surface of the Al2O3 oxide film was smooth without cracks, and there was no obvious MoO3 phase, so that the Mo(Si,Al)2 coatings had a better antioxidant performance in the mid-temperature stage. 9Si-8B alloy surface using a combination of magnetron sputtering and subsequent annealing to prepare two Mo(Six,Al1-x)2 high temperature protective coatings. After magnetron sputtering, the inner layer of both coatings was a 2 μm Mo-12Si-21B diffusion barrier, and the outer layer was a 5 μm antioxidant coating consisting of Mo-48Si-24Al and Mo-71Si-8Al, respectively. After vacuum annealing treatment, the inner layer was transformed into Mo5SiB2 phase, and both outer layers were transformed into Mo(Si,Al)2 and MoSi2 phases.The results of cyclic oxidation experiments of the two coatings and the substrate in air at 800°C and 1000°C showed that the oxidized mass loss of the coated samples was reduced dramatically in comparison with that of the substrate.After oxidation of the Mo-48Si-24Al coatings at 800°C, the surfaces formed a layer-like oxide film, the inner layer consists of SiO2 and Al2SiO5 or 3Al2O3-2SiO2 mullite phases, and the outer layer is acicular 9Al2O3-2B2O3 phase; whereas the Mo-71Si-8Al coating did not form acicular boron-aluminate phases after oxidation at 800 °C. Both coatings can form an oxidative protection against the substrate for up to 100 h at 1000 °C, and the oxide films are both composed of dense SiO2 and mullite phases.
It has been shown that the addition of Ti element also helps to improve the oxidation resistance of MoSi2 coatings [35-36].Li et al. [36] prepared a new MoSi2/(Mo,Ti)Si2 biphasic composite coating on the surface of molybdenum alloy by slurry sintering method. The coating has a bilayer structure, with the outer layer consisting of tetragonal C11b MoSi2 phase and hexagonal C40(Mo,Ti)Si2 phase, and the inner layer is a diffusion layer of (Mo,Ti)5Si3. The substitution of Mo by Ti causes part of MoSi2 to be transformed from the tetragonal C11b phase to the hexagonal C40(Mo,Ti)Si2 phase. The composite coating provides better oxidation protection for the molybdenum substrate at 1500 °C, which is due to the formation of a Si-Ti-O composite glassy oxide film on the surface after oxidation of the coating, which can effectively inhibit the internal diffusion of oxygen. At 1600°C, the antioxidant performance of the coating decreases due to the crystallization of part of the amorphous SiO2, which leads to the change of its volume and the appearance of microcracks in the SiO2 oxide film, and thus the antioxidant performance of the coating decreases. He Haoran et al [37] prepared (Ti,Mo)Si2/MoSi2 composite coatings in situ on molybdenum surfaces by the embedded infiltration method. Two processes were selected for the embedded infiltration process, namely, one-step co-deposition method and two-step deposition method. The results showed that the co-deposition method could not obtain a continuous (Ti,Mo)Si2 layer, while the two-step deposition process of infiltrating titanium and then silicon could obtain a continuous and dense composite coating. The composite coating consists of (Ti,Mo)Si2 layer, MoSi2 layer, Mo5Si3 layer and matrix from outside to inside, and its formation is mainly controlled by the diffusion of Ti and Si. Single MoSi2 coating at 1200 ℃ cyclic oxidation 120h after the beginning of rapid mass loss, 140h after the complete failure; while the composite coating oxidation 180h after no significant mass loss, the surface of the dense composite oxide film composed of SiO2 and TiO2 can inhibit the “pest” oxidation and improve the antioxidant performance of the coating. The antioxidant performance of the coating.
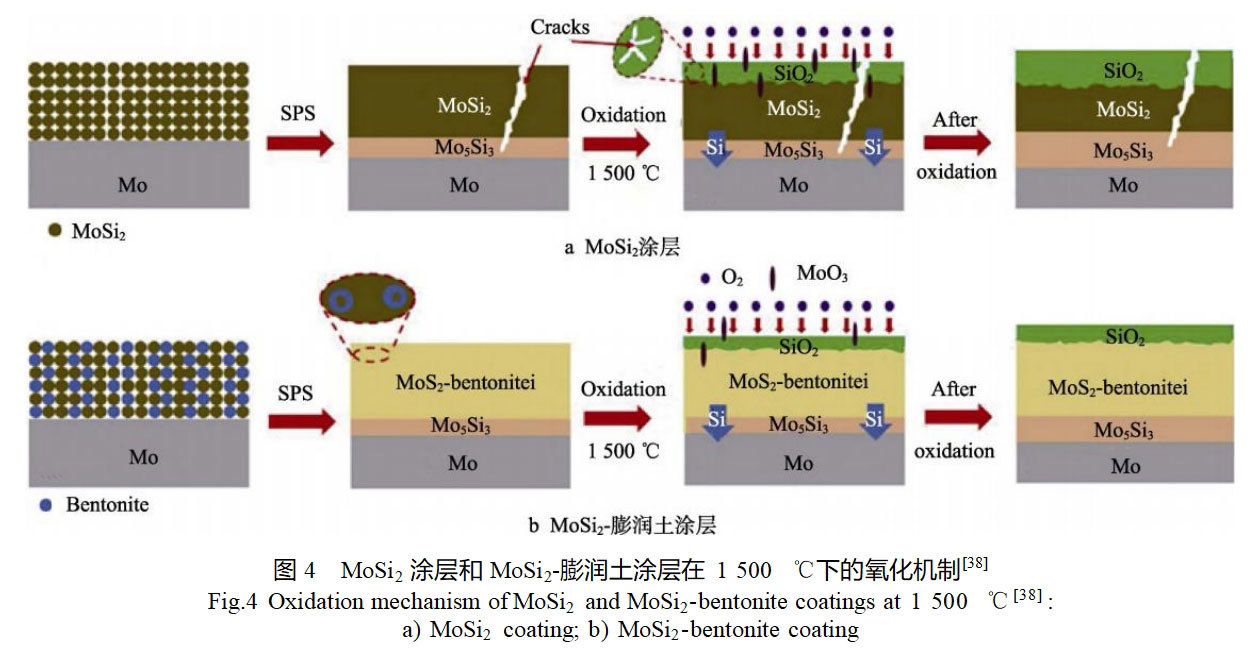
In addition to reactive elements such as B, Al, and Ti, reinforcing phases with lower coefficients of thermal expansion, such as bentonite, YSZ, and Si3N4, have been added to the MoSi2 coatings to alleviate the difference in the coefficients of thermal expansion between the coatings and the substrate [38-40].Zhu et al [38] used discharge plasma sintering to prepare the MoSi2 coatings and the bentonite doped MoSi2 coatings on the surface of a molybdenum substrate , and the phase composition, morphology and high temperature oxidation properties of the two coatings were comparatively studied. The results show that both coatings are mainly composed of MoSi2 and Mo5Si3 phases, and a small amount of Al2O3 phase exists in the MoSi2-bentonite composite coating.The structure and oxidation process of the two coatings are shown in Fig. 4, and there are penetrating cracks in the MoSi2 coating, while there are no cracks in the MoSi-bentonite composite coating, which is mainly due to the fact that the bentonite clay can effectively reduce the thermal conductivity between the MoSi2 coating and the molybdenum matrix. and molybdenum matrix. Compared with the MoSi2 coating, the composite coating exhibits better resistance to high-temperature oxidation, with a thinner SiO2 oxide film and Mo5Si3 diffusion layer formed after oxidation. They [41] also prepared a multilayer MoSi2-based coating consisting of a bonding layer, a protective layer and an oxygen diffusion barrier layer on the surface of a molybdenum substrate sequentially by a three-step dip-coating process using industrial MoSi2 waste as raw material, and pressureless sintered the coating at elevated temperatures, and also chose a pure MoSi2 powder raw material for comparison. The results show that the MoSi2-based coatings prepared from waste materials have no crack defects, which is mainly due to the fact that their coating composition contains bentonite and other low thermal expansion coefficient phases, which can effectively alleviate the difference in thermal expansion coefficients between the coatings and the substrate.After oxidization of the two types of coatings at 1500 ℃ for 20 h, the coatings prepared from MoSi2 wastes have lower oxidation mass increment, and their surface formed SiO2 oxide film is continuous and dense with lower porosity.
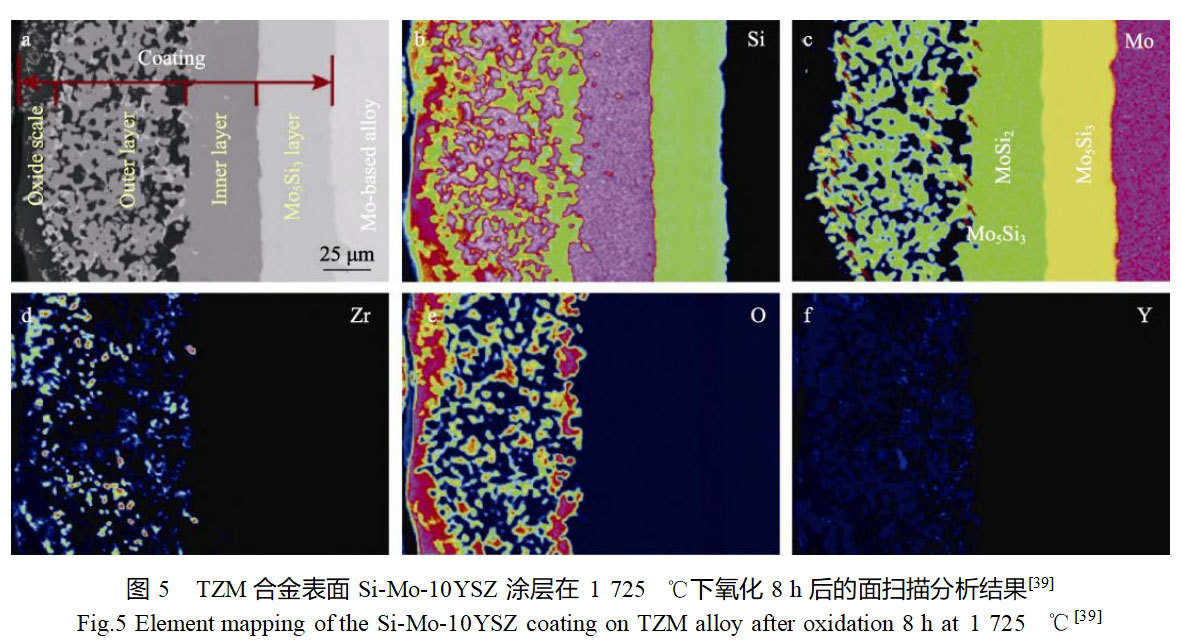
Cai et al [39] prepared a novel YSZ-modified silicide coating on the surface of TZM alloy by slurry spraying and reaction sintering method, and investigated the effect of YSZ content on the organization and oxidation properties of the coating.The Si-Mo-YSZ coating consisted of a MoSi2-ZrSi2-SiO2 outer layer and a MoSi2 inner layer.The results of the static high-temperature oxidation experiments at 1725°C showed that the TZM alloy oxidized for 0.35h is completely failed, while the protection time of Si-Mo-YSZ coating with YSZ mass fraction of 0%, 5%, 10% and 15% on the substrate is 8.6, 13.5, 22.3 and 14.2h, respectively, which proves that all the modified coatings can protect the molybdenum alloy substrate efficiently, and the anti-oxidation ability of Si-Mo-10YSZ coating is the best, which is mainly The Si-Mo-10YSZ coating was oxidized at 1725°C for 8 h. After oxidizing at 1725°C for 8 h, a dense and continuous SiO2 oxide film was formed on the surface, in which a certain amount of ZrO2 and ZrSiO4 phases were also diffusely distributed, as shown in Fig. 5. The high melting point of ZrO2 and ZrSiO4 phases helps to improve the melting point and heat resistance of the SiO2 oxide film, and the thermal expansion coefficient of the composite oxide film is higher than that of the single SiO2 oxide film, which can alleviate the difference in thermal expansion coefficients between the oxide film and the coating. In addition, the oxygen transmission rate in the ZrSiO4-ZrO2-SiO2 composite oxide film is lower than that in the single SiO2 oxide film, which can reduce the rate of Si consumption in the coating and thus extend the protection time of the coating.Zhang et al [40,42] prepared (Mo,W)Si2-Si3N4 composite coatings on the surface of molybdenum substrate by three-step embedded infiltration method and The high-temperature oxidation properties of MoSi2-Si3N4, MoSi2-CrSi2-Si3N4 and (Mo,W)Si2-Si3N4 composite coatings at 1600 °C were comparatively investigated. The results show that (Mo,W)Si2-Si3N4 composite coatings are well combined with the substrate, the coating organization has a columnar crystalline structure, and the phenomenon of accelerated mass loss only begins to appear after oxidation at 1600 ℃ for 360 h. Its oxidation protection time against molybdenum substrate is 3.75 and 5 times that of the MoSi-CrSi2-Si3N4 and MoSi2-Si3N4 coatings, respectively. During the oxidation process, a SiO2 oxide film was formed on the surface of the (Mo,W)Si2-Si3N4 composite coating, and (Mo,W)5Si3 and (Mo,W)3Si transition layers were formed between the coating and the substrate. The service life of the silicide-based coatings is closely related to the Mo5Si3 transition layer formed by their oxidation, so they also comparatively studied the growth rates of (Mo,W)5Si3 or Mo5Si3 layers in the oxidation process of the three coatings at 1600 ℃. The thickness changes of the transition layer in the three coatings follow the parabolic law, and the growth rate of the transition layer of the (Mo,W)Si-Si3N4 coating has the lowest constant, which proves that the growth rate of the transition layer of W element is the lowest. constant is the lowest, which proves that the introduction of W element has a strong hindering effect on the diffusion of Si between the coating and the molybdenum matrix.
3.1.3 Silicide-based gradient composite coatings
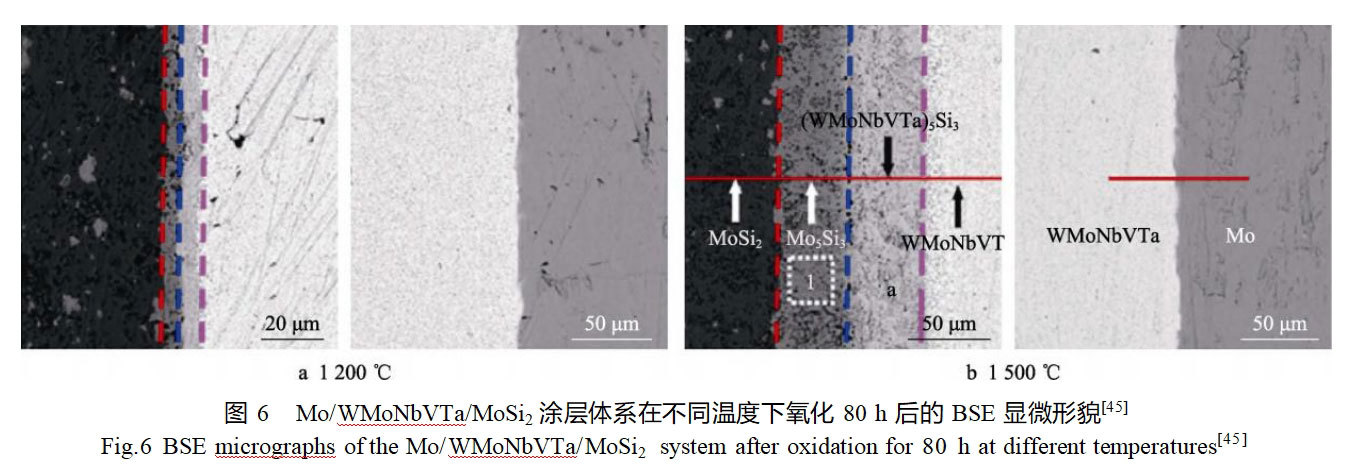
The structural design of the coating is mainly based on the gradient change of the coating composition or the preparation of a diffusion barrier layer to alleviate the difference in thermal expansion coefficients between the coating and the substrate and to inhibit the diffusion of active components between the coating and the substrate.Liu et al [43] prepared a MoSi2/SiC-Mo2C gradient composite coating on the surface of molybdenum substrate in situ using two-step embedded infiltration method, in which the molybdenum substrate was first carburized in graphite to obtain a Mo2C layer, and then the molybdenum matrix with Mo2C layer was siliconized in silicon powder to obtain MoSi2/SiC layer. They simultaneously investigated the cyclic oxidation behavior of the composite and single MoSi2 coatings at different temperatures and discussed the effect of the Mo2C barrier layer on the oxidation resistance of the coatings. The results show that no “pest” oxidation occurs in both composite and single MoSi2 coatings after oxidation at 500 °C for 100 h. The antioxidant properties of the composite coatings are better than those of the single MoSi2 coatings at high temperatures (1200, 1400, 1600 °C). The SiC phase in the MoSi2/SiC-Mo2C composite coating can slow down the difference in thermal expansion coefficient between the MoSi2 coating and the molybdenum substrate, while the Mo2C barrier layer plays a key role in slowing down the diffusion of C and Si into the molybdenum substrate, and thus the composite coatings have a better antioxidant performance. On the basis of MoSi2/SiC-Mo2C composite coating, Jiang et al [44] successfully prepared CrSi2/MoSi2/SiC-Mo2C gradient composite coatings on the surface of molybdenum matrix by using a three-step embedded infiltration method, with the outer layer of the coating consisting of CrSi2, MoSi2, and SiC, and the inner layer of Mo2C layer. By comparing with the MoSi2/SiC-Mo2C coating system, the mechanism of the influence of the Cr element in the composite coating on the antioxidant properties was emphasized. The results show that the antioxidant capacity of the 2 coatings is close at 500 °C, while the CrSi2/MoSi2/SiC-Mo2C coating presents better antioxidant performance at 1600 °C temperature. The Cr2O3 formed by the oxidation of Cr elements in the coating not only alleviates the difference in thermal expansion coefficients between the coating and the substrate, but also helps to accelerate the formation of the mixed protective oxide film at low temperatures. In addition, Cr2O3 in the oxide film can inhibit the volatilization of SiO2 at high temperatures and improve the stability of the oxide film in the glassy state.Yan et al [45] investigated the effect of the diffusion barrier layer of WMoNbVTa high-entropy alloy on the high-temperature oxidation performance of the MoSi2 coating on the surface of molybdenum substrate. They prepared a double-layer coating on the surface of molybdenum matrix by discharge plasma sintering method, with an inner WMoNbVTa layer and an outer MoSi2 layer, and comparatively investigated the interdiffusion behaviors of the Mo/WMoNbVTa/MoSi2 and Mo/MoSi2 coatings at 1200~1500℃. After prolonged oxidation, two diffusion layers such as Mo5Si3 and (WMoNbVTa)5Si3 appeared at the WMoNbVTa/MoSi2 interface, while no obvious diffusion layer appeared at the Mo/WMoNbVTa interface, as shown in Fig. 6. The growth rate of the diffusion layer in the Mo/WMoNbVTa/MoSi2 coating is much lower than that in the Mo/MoSi2 coating, which proves that the WMoNbVTa high-entropy alloy layer can effectively inhibit the elemental interdiffusion between the molybdenum substrate and the MoSi2 coating.
3.2 Aluminide coating
Aluminum oxide coating belongs to the heat diffusion coating, aluminum and oxygen have high affinity, in the high temperature oxidation environment can be formed on the surface of the coating of dense Al2O3 oxide film, can effectively block the internal diffusion of oxygen. Aluminum oxide coating preparation process is simple, suitable for static isothermal environment in the oxidation protection; but its high temperature mechanical properties are poor, when subjected to thermal shock, the coating is prone to defects and spalling, which ultimately leads to coating failure.
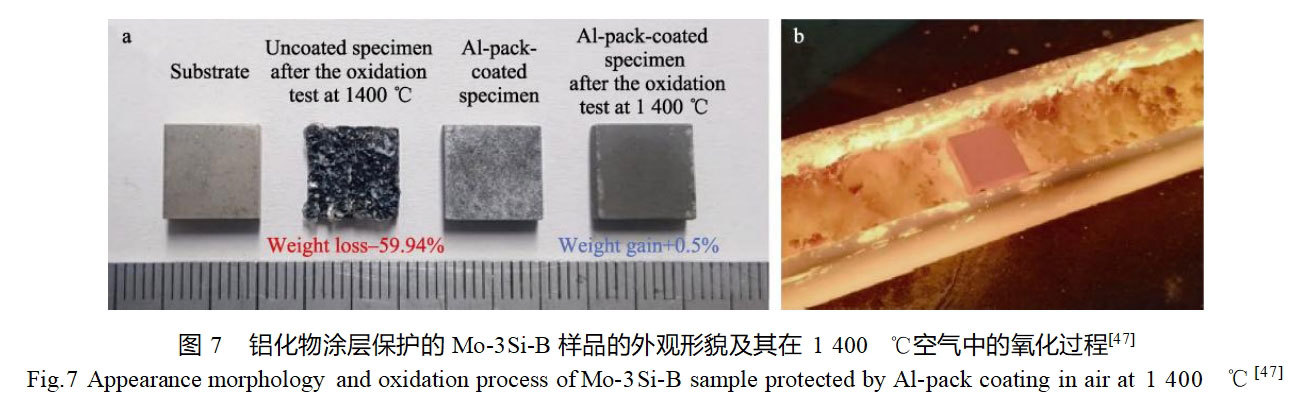
Chakraborty et al [46] prepared an aluminide coating on the surface of TZM alloy using the encapsulated infiltration method, and investigated the effects of infiltrant composition and reaction temperature on the quality and thickness of the coating. The bilayer structure of aluminide coating with thickness of 50 μm can be obtained on the surface of TZM alloy after 12 h of reaction at 1000 ℃, and its physical phase compositions are Al5Mo, Al7Mo4 and Al2(MoO4)3. The mass of the aluminide coating increases rapidly due to the generation of Al2O3 at the initial stage of oxidation at 1000 and 1200 ℃, and the oxidation rate becomes slower due to the formation of the protective Al2O3 oxide film. Choi et al [47] prepared aluminide coatings on the surface of Mo-3Si-1B molybdenum alloy using NH4Cl, Al and Al2O3 as raw materials by the embedded infiltration method and investigated the growth kinetics and isothermal oxidation behavior of the coatings. The aluminide coatings consisted of MoAl4, Mo3Al8 and a diffusely distributed precipitated phase. They estimated the equilibrium rate constant k0 and activation energy Q for the growth of the aluminide coatings based on the experimental results with different embedding and infiltration treatment times at temperatures of 800, 900, and 1000 °C, and established a model for the growth kinetics of the coatings. The coating thickness estimated by the model is basically consistent with the experimental results, which proves the reasonableness of the model. The high-temperature oxidation performance of Mo-3Si-1B substrate and alumina coating in air at 1400°C was also compared, as shown in Fig. 7. The substrate showed a mass loss of 59.94% after 5 h of oxidation, while the coating showed only a slight mass increment of 0.5% after 36 h of oxidation, and its excellent antioxidant performance was mainly related to the protective α-Al2O3 oxide film formed by oxidation.Park et al [48] comparatively studied the high-temperature oxidative properties of TZM alloys and TZM alloys with alumina coatings under dynamic plasma flame. The aluminide coating was made by the embedded infiltration method, and its thickness was about 200 μm, mainly composed of Al3Mo and Al8Mo3 phases.The TZM alloy lost 80% of its mass after exposure to a dynamic plasma flame environment for 4 min, while the aluminide-coated samples showed almost no change in mass after 8 min and 30 s of flame ablation, and at the same time, there was no change in the thickness of the coating. Therefore, the aluminide coating has the possibility to serve in dynamic high temperature oxidation environment.
3.3 Heat-resistant alloy coating
Heat-resistant alloy coatings are developed on the basis of nickel- and cobalt-based high-temperature alloys, and their antioxidant mechanism is to utilize the dense oxide film formed on the coating surface to impede the diffusion of metal cations. For example, adding Cr to nickel- or cobalt-based alloys can significantly reduce the oxidation rate of the alloy matrix, which is due to the fact that Cr will participate in the formation of a dense CoCr2O4 or NiCrO4 spinel-type oxide layer, thus improving its antioxidant capacity.Huang et al [49] deposited a Ni-20Cr alloy coating on the surface of molybdenum using laser fusion curing technology, and the coating was tightly bonded with the substrate and There were no defects such as cracks and holes. Due to the partial dilution of the substrate and convective movement in the melt during the cladding process, the Mo element in the substrate partially enters into the coating, and thus the coating mainly consists of three elements such as Ni, Cr, and Mo. The quality change of the coating oxidized in static air at 600℃ for 100h is very small, which can form an effective protection for the substrate. The oxide film on the surface of the coating after oxidation consists of NiO, Cr2O3 and NiMoO4, and the continuous dense Cr2O3 layer can effectively prevent further oxidation of the coating and the substrate. Although the heat-resistant alloy coating has good high-temperature oxidation resistance, but Ni and molybdenum matrix interdiffusion will affect the performance of the molybdenum matrix, and there is a thermal stress mismatch between the coating and the substrate, so there are limitations in the application of this type of coating.
3.4 Oxide coating
Inert oxides have good high temperature stability, not easy to chemical reaction with the substrate material, in the substrate surface can play the role of oxygen diffusion barrier layer. Inert oxides are usually combined with silicate glass to form an oxide-glass based antioxidant coating. In order to prolong the oxygen diffusion path, the inert oxides, which are used as antioxidant coatings, usually have a large thickness, so the focus should be on the preparation process of the coatings during the coating design to avoid defects such as cracks in the coatings.
Wang Zhiyong [50] prepared a new silicate coating on the surface of molybdenum substrate by slurry sintering method, which used barium silicate glass as a continuous phase and ZrO2 as a refractory filler. The thermal expansion coefficient of the coating and the substrate was closest when ZrO2 was added at 20%, and the formed coating had a flat surface and was tightly bonded to the substrate. After oxidizing at 1000°C and 2h, the mass loss of the coating sample was 31.13 mg/cm2, which has good oxidation resistance. It was also found that the antioxidant capacity of the coating treated by pre-firing at 1300 °C for 20 min was significantly improved, and its mass loss was 42.13 mg/cm2 after 1000 °C and oxidation for 20 h. Pengfei Zhu et al [51] prepared Y2O3-glass-based coatings on the surface of a molybdenum substrate, and investigated the effect of different Y2O3 contents (mass fractions of 0%, 10%, 20%, and 0%) on the antioxidant performance of the coating. ) on the antioxidant properties of the coatings. The silicate glass matrix was mainly composed of BaO, SiO2 and small amounts of B2O3, ZnO, CaO and Al2O3. When the Y2O3 addition was 10%, the coating had the best resistance to high-temperature oxidation at 1200℃. Coating oxidation in the outermost layer formed a layer of dense SiO2 layer, at the same time the coating of BaO can be oxidized with the molybdenum matrix MoO3 reaction generated BaMoO4. BaMoO4 in the SiO2 layer below the formation of a layer of dense, uniform viscous molten state inert protective film, to further isolate the oxygen and the substrate.
4 coating preparation technology
At present, molybdenum and molybdenum alloy surface high-temperature protective coatings commonly used preparation methods include slurry sintering method, embedded seepage method, plasma spraying method, molten salt method, chemical vapor deposition method, magnetron sputtering method and so on.
4.1 Slurry sintering method
Slurry sintering method is the source of penetration and binder, activator, solvent and other additives in accordance with a certain proportion of ball milling and mixing to make a slurry, and then the slurry will be sprayed or brushed on the surface of the substrate and dry, and then placed in a vacuum or an inert atmosphere for high-temperature sintering, and ultimately to obtain the desired coating [52]. The slurry sintering method has the advantages of low cost, simple process, and tight bonding between the coating and the substrate, but the controllability of the coating thickness is poor. Cai et al [39] prepared Si-Mo-YSZ coatings on the surface of TZM alloys using a two-step method of slurry spraying and reaction sintering. The coating preparation process is shown in Fig. 8. Firstly, the raw materials Si-Mo, Mo, YSZ powder and a small amount of NH4F, SiO2 and PVB were dispersed into ethanol solvent, and then the slurry was obtained after ball milling treatment, and the slurry was sprayed onto the surface of TZM alloy substrate by using an air compressor and a spray gun. After the slurry was dried, the TZM alloy coated with the slurry was placed in a vacuum sintering furnace, and the temperature was first raised to 500°C and held for 1 h to remove water and PVB, and then sintered in a holding temperature of 1450°C for 1 h. During the sintering process, the molten Si in the slurry chemically reacted with Mo and ZrO2 to produce MoSi2, ZrSi2, and SiO2, which constituted the main components of the coating. Xiao Lairong et al [52] prepared MoSi2-ZrB2 high temperature antioxidant coating on the surface of molybdenum alloy by slurry sintering method. The slurry was prepared by mixing MoSi2, ZrB2 and homemade sintering agent in a certain ratio, and then adding ethanol and binder ball milling for 10h. The slurry is coated on the surface of the substrate, dried in a vacuum drying oven for 2h, and then vacuum sintered at 1580 ℃ for 30min. the surface of the coating is mainly MoSi2, ZrB2 and a small amount of SiC, the coating is dense and uniform, and there are no obvious defects, the thickness of about 100 μm. the coating's oxidation resistance life in the 1670 ℃ static air is up to 12h.
4.2 Embedded infiltration method
Embedded percolation belongs to the in-situ chemical heat treatment technology, which is to bury the substrate to be plated into the percolating agent composed of active substances, halide active agents and inert fillers, and then heated for a period of time in a closed container under a vacuum or protective atmosphere, through the decomposition and adsorption process of the active substances, so that the active elements continue to diffuse to the substrate, and then in-situ reaction occurs on the surface of the substrate to form a certain thickness of the coating. The preparation process of embedded infiltration is relatively simple [53], and the metallurgical bonding between the coating and the base metal results in a high bonding strength, which is not easy to peel off. Depending on the infiltrated elements, coatings of different compositions can be obtained on the surface of the substrate. Meanwhile, the composite coating can be prepared by multi-step deposition or one-step co-deposition according to the design of the infiltrant composition and the embedded infiltration process.
Sun et al [54] prepared MoSi2 coatings by halide-activated embedded infiltration and investigated the effects of different fillers (Al2O3, SiO2, SiC) on the microstructure and antioxidant properties of the coatings. The halide activation embedded infiltration process is an in-situ diffusion reaction coating preparation process that occurs on the surface of the substrate, as shown in Figure 9. During the heating process, NH4F as the activator reacts with the high-purity silicon powder in the embedded powder to generate gaseous SiFx (1<x<4), which is transported to the substrate surface through the powder interstices and undergoes a decomposition reaction on the surface of the substrate to generate reactive atoms [Si]. Subsequently, the reactive atom [Si] undergoes adsorption on the substrate surface and undergoes a diffusion reaction with the substrate to produce a silicide. The infiltration of [Si] leads to a higher surface energy on the coating surface, and therefore the filler is absorbed into the coating during the cooling process. The results show that the coatings prepared from Al2O3 and SiO2 fillers exhibit a bilayer structure consisting of an outer layer of MoSi2 and an inner layer of Mo5Si3, whereas the coatings prepared from SiC fillers consist only of MoSi2. The surface of the coatings prepared from SiO2 fillers was rougher and had more residual SiO2 fillers embedded in the surface of the coatings compared to the coatings prepared from Al2O3 or SiC fillers. After oxidation at 500 °C for 110 h, all three coatings showed good antioxidant properties, which can effectively protect the molybdenum substrate against “pest” oxidation. After oxidation at 1200°C for 110h, the coating prepared by SiO2 filler showed the best antioxidant ability, with the quality change of only 0.05%.
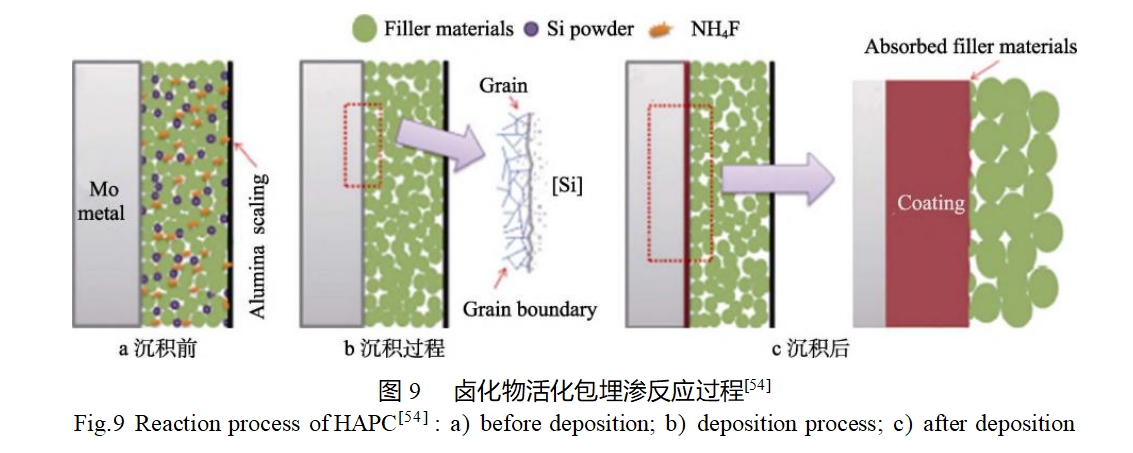
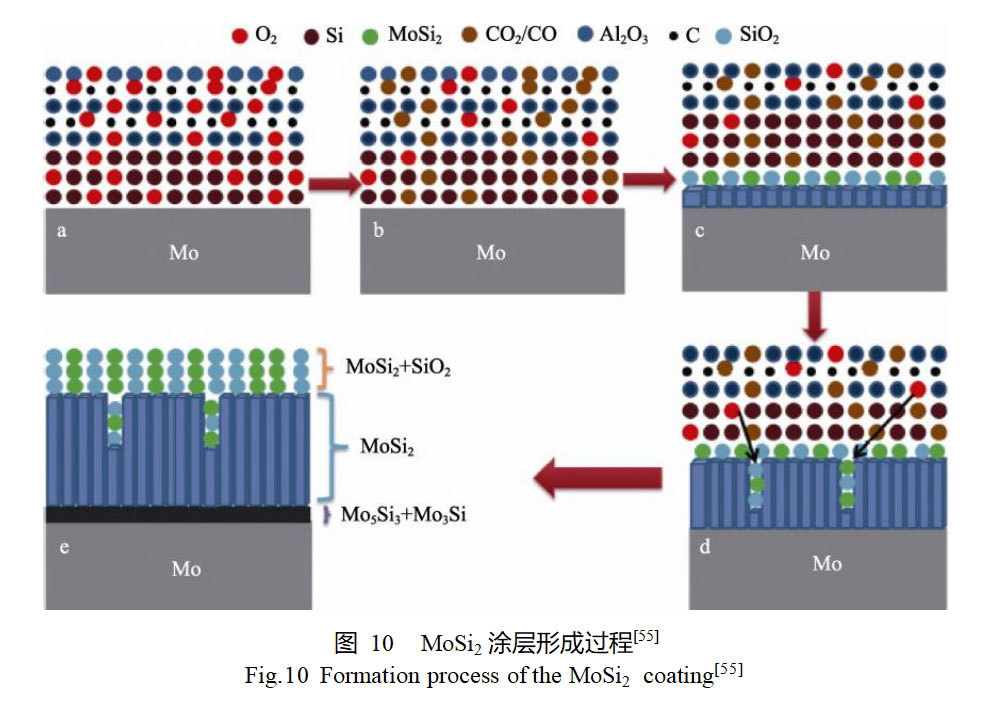
Conventional embedded infiltration is usually carried out under vacuum or inert atmosphere protection. yang et al [55] improved the embedded infiltration process by adding carbon powder to the filler to make it consume the oxygen in the environment, and realized the in-situ preparation of MoSi2 coatings on the surface of molybdenum substrate under the atmospheric condition. the process of the formation of MoSi2 coatings is shown in Fig. 10. In the initial heating stage, the carbon powder in the penetrant reacted with oxygen to form CO or CO2, which reduced the oxygen partial pressure in the system, but the oxygen was not completely consumed. With the continuous generation of MoSi2, a small amount of residual oxygen was able to react with MoSi2 to form SiO2, which could be filled into the coating interstices, thus facilitating the formation of a denser coating. It is found that the prepared MoSi2 coating has excellent antioxidant properties, and the coating remains intact after oxidation at a high temperature of 1600°C for 1 h. The glassy SiO2 layer formed by surface oxidation can effectively protect the coating and substrate underneath.
4.3 Plasma spraying method
Plasma spraying belongs to a kind of thermal spraying technology, spraying powder is heated to molten or semi-molten state and accelerated at the same time under the action of plasma jet, and then flies to the substrate at a certain speed, and deposits on the surface of the substrate to form a coating [56]. The coatings prepared by plasma spraying have characteristic laminar structure and small thermal deformation of the substrate, so they are widely used in surface repair of workpieces [57-59].
Wang et al [60] used atmospheric plasma spraying technology to prepare MoSi2 protective coatings on the surface of molybdenum substrate, and systematically investigated the effects of spraying process parameters such as spraying power, argon flow rate, and spraying distance on the phase composition, microstructure and properties of the coatings. Under different spraying process parameters, the coatings were composed of MoSi2(t) with tetragonal crystal structure, MoSi2(h) with hexagonal crystal structure and Mo5Si3(t) with tetragonal crystal structure. The microstructure of the coating strongly depends on the spraying process parameters. During the spraying process, the molten MoSi2 particles collide with the substrate at a certain speed, and the coating formation process is simultaneously affected by the speed of the colliding particles, the particle size, the molten state, and the surface state of the substrate or the formed coating. With the increase of spraying power or the decrease of argon flow rate, the microhardness and bonding strength of the coating gradually increase, and the porosity gradually decreases. This is because increasing the spraying power or reducing the argon flow rate can increase the temperature of the plasma flame, the molten proportion of the sprayed powder increases, the latter impact to the surface of the substrate can be fully spread, and form a good combination with the substrate [16].
4.4 Molten salt method
Molten salt method is to alkali metal or alkaline earth metal halide salt as a reaction medium, the reactants and salt according to a certain proportion of the preparation into a molten salt mixture, then heated to make it melt, and then the substrate is immersed in the high temperature molten salt, the reactants in the molten salt medium to react, so as to form a coating on the surface of the substrate [61]. According to the different reaction mechanisms, the molten salt method can be divided into non-electrochemical and electrochemical methods. The non-electrochemical is similar to the encapsulated infiltration method, which mainly utilizes the in-situ reaction of the active components on the substrate surface; the electrochemical method is to deposit the reactants on the substrate surface by electrochemical reduction through the application of a voltage or an electric current during the reaction process. The molten salt method is faster than the encapsulated infiltration method in terms of deposition rate and hence higher productivity, with the disadvantage of non-uniform coating thickness [62].
Dai et al [63] prepared MoSi2 coatings in situ by electrodeoxygenation of a prefabricated SiO2 layer on the surface of a molybdenum substrate in molten calcium chloride at 900 °C, and discussed the effects of tank voltage and temperature on the anodic reduction products. The results show that the reduction rate of electrodeoxygenation increases with the increase of the tank voltage and the molten salt temperature; when the tank voltage is 3.1 V and the electrolysis time is 3 h, a dense MoSi2 coating can be obtained. The study on the formation mechanism of MoSi2 coating shows that the SiO2 layer is reduced to form Mo-Si alloy on the surface of molybdenum substrate, and at the same time, CaSiO3 and Ca3Si2O7 are also formed with Ca2+ and O2- in the molten salt, and the remaining SiO2 and CaSiO3 and Ca3Si2O7 are reduced to Mo-Si alloy, and ultimately the Si diffusion in the molybdenum substrate is reduced to form Mo-Si alloy. MoSi2 coatings were formed on the molybdenum surface with the diffusion of Si. Wang et al [64] prepared MoSi2 coatings and Si-MoSi2 gradient silicide coatings on the molybdenum substrate surface by electrodeposition at 800 °C in the NaCl-KCl-NaF-K2SiF6 molten salt system. The results showed that the cathodic current density had a significant effect on the type and thickness of the coatings, and the final type of the coatings depended on the competition between the reduction rate of Si and the diffusion rate of Si into the MoSi2/Mo interface. Also, they investigated the electrochemical behavior of Si ions in fluorochlorine molten salts using cyclic voltammetry and chronopotentiometry to reveal the mechanism of their electroreduction.The reduction of Si(IV) to Si was shown to be quasi-reversible or irreversible diffusion-controlled single-step reaction.Kuznetsov et al [65] prepared boride and silicide coatings on molybdenum substrate surfaces by molten salt method, respectively. The boriding process was carried out at 850-1050°C, in the NaCl-KCl-KBF4-NaF-B molten salt system, and the boride coatings were prepared under the conditions of no applied current and applied current (5-10 mA/cm2), respectively. The boride coatings prepared using diffusion boriding are mainly composed of Mo2B and MoB phases, which have poor antioxidant properties; whereas, thicker boride coatings can be obtained using electrochemical boriding, and coatings containing Mo2B5 phase can be prepared at conditions higher than 950 °C and 10 mA/cm2. The Mo2B5-containing coating samples exhibited better oxidation resistance at 500 °C in an aqueous air environment. The preparation process of silicides is similar to that of borides, and at the same reaction temperature, the silicides coatings prepared by electrochemical method are thicker compared to diffusion infiltration of silicon.
4.5 Chemical vapor deposition
ChemicalVaporDeposition (CVD method) is a process of forming coatings by chemical reaction of gaseous reactants containing coating elements on the surface of the substrate in a reaction chamber at a certain vacuum and temperature. The coating prepared by this method has a dense and uniform organization, and the composition is flexible and controllable, which is suitable for the preparation of coatings on the surface of complex shaped substrates. The disadvantages are the low deposition efficiency and the toxicity of the gas source and most of the reaction by-products [66].
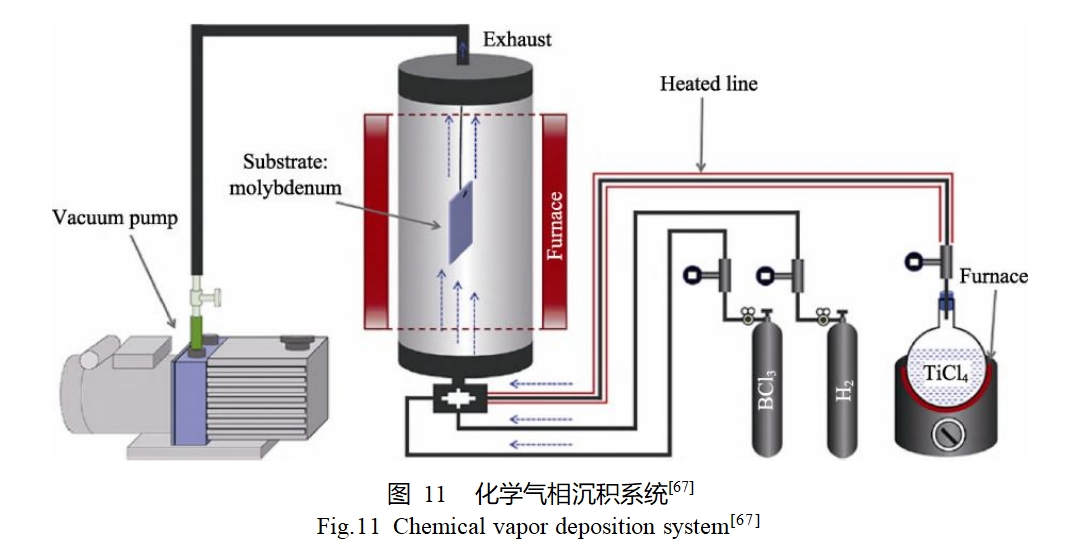
Huang et al [67] prepared TiB2 ceramic coatings on the surface of molybdenum substrate using CVD method. The preparation process is shown in Fig. 11, where the molybdenum substrate was placed in the center of the deposition chamber, and the gaseous TiCl4, BCl3, and H2 precursors were passed through the inlet line into the deposition chamber, respectively. The deposition pressure was controlled at 40~50 kPa and the temperature was 1000 °C. During the reaction, TiCl4 and BCl3 were reduced to TiB2 by H2. After 2 h of deposition, the thickness of the TiB2 coating was up to 13 μm, and the coating showed a typical columnar crystalline structure and was well bonded to the substrate.Yoon et al [68] summarized the reaction mechanism of preparing MoSi2 coatings on molybdenum surfaces by CVD method. mechanism, using SiCl4 and H2 as the reaction gases, the reaction is divided into the following four steps: mass transfer of the reaction gas from the main gas stream to the substrate surface through the gas boundary layer; the reaction of gas molecules being adsorbed on the substrate surface and deposition of Si on the surface; the solid-state diffusion of Si into the substrate; and the reaction of Si with the Mo substrate to produce MoSi2.
4.6 Magnetron sputtering
Magnetron sputtering belongs to a physical vapor deposition technology, it is the use of energy-carrying particles to bombard the surface of the target material, so that the target surface atoms from the original lattice overflow, and with a certain amount of energy to fly to the surface of the substrate and film. Magnetron sputtering has the advantages of high coating purity, fast film formation, and large-area coating preparation [69-71].
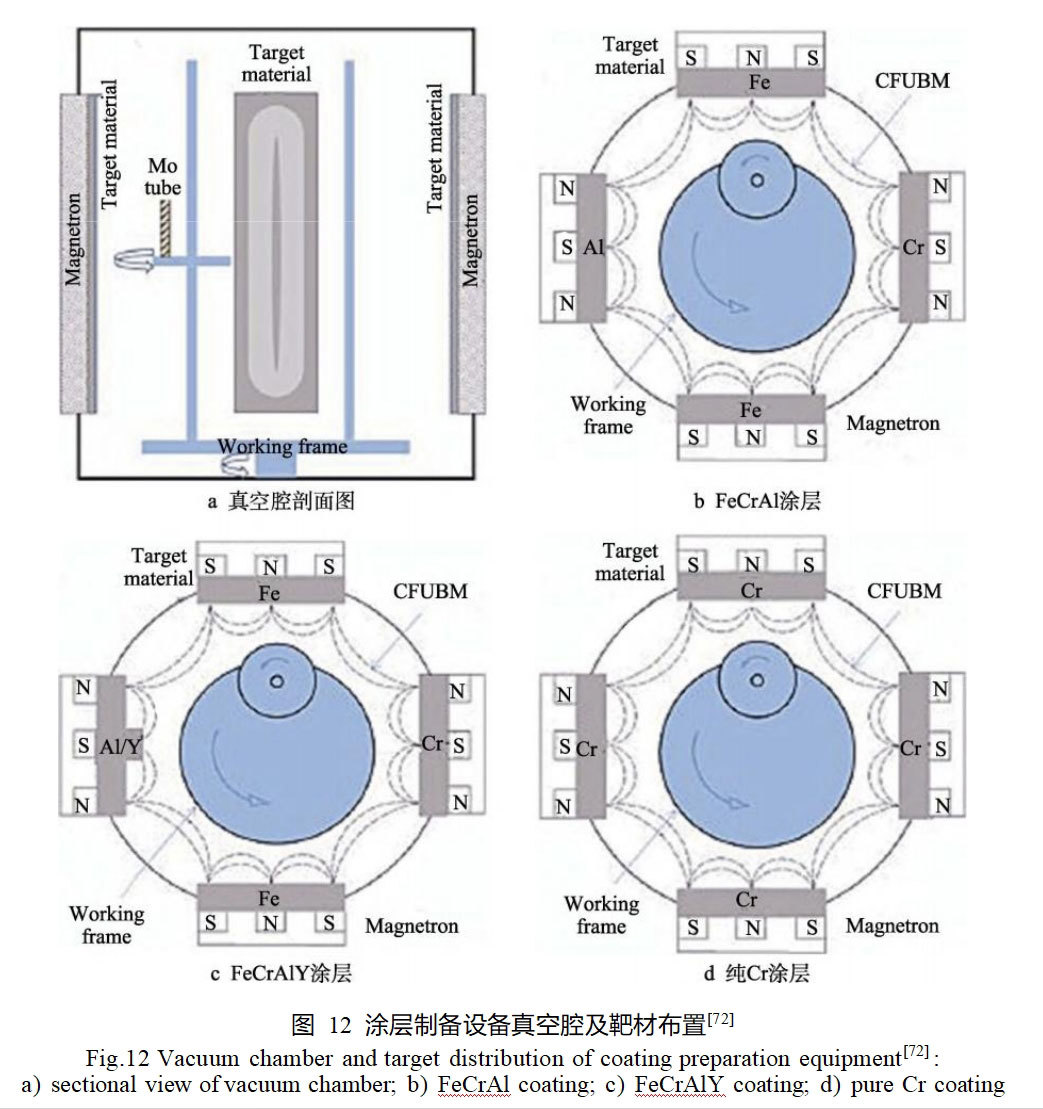
Tingting Li [72] prepared FeCrAl, FeCrAlY and pure Cr coatings on the surface of molybdenum alloys by magnetron sputtering technique, respectively. The schematic diagram of magnetron sputtering device and target arrangement is shown in Fig. 12. By regulating the target current of different target materials, the incident particle current density of Fe, Cr, Al, Y and other elements can be controlled, which in turn realizes the control of the content of each component in the coatings.The thicknesses of the three coatings are similar, and the cross-sectional morphology shows the typical columnar crystalline structure.The hardness of the FeCrAl and FeCrAlY coatings, which are mainly composed of FeCr solid solution, is greater than that of the pure Cr coatings, and the bonding strength of the pure Cr coatings with the substrate is better than that of the pure Cr coatings. The bonding strength with the substrate is better than that of the other two coatings.Lange et al [69] used a combination of magnetron sputtering and subsequent annealing to prepare three Mo-Si-B bilayer coatings on the surface of Mo-9Si-8B alloy. Firstly, a 2 μm thick amorphous Mo-12Si-28B layer was deposited on the substrate surface using a Mo target and a SiB2 target at -30 V bias and 108 °C, and the Mo-12Si-28B layer was annealed and treated for 2 h in a vacuum furnace at 900 °C in order to obtain the Mo5SiB2 layer. Subsequently, four Mo-Si-B coatings with different compositions were magnetron sputtered on the surface of the annealed layer.SiB2 target, Si target, Mo target, and B target were used to prepare Mo-55Si-10B and Mo-29Si-15B coatings, and SiB2 target, Mo target, and two Si targets were used to prepare Mo-45Si-25B coating. After vacuum treatment, the four outer coatings underwent crystallization transformation to produce MoSi2, Mo5Si3 and MoB phases.
5 Problems and outlook
Although positive progress has been made in the research of high-temperature protective coatings on the surface of molybdenum and molybdenum alloys at home and abroad, there is still a need to further optimize the existing coating systems and actively develop new coating systems or preparation processes so that the coatings can provide antioxidant protection to the substrate for a long time over a wide range of temperatures to meet the needs of practical application. The future can be considered around the following directions to carry out related research work:
(1) Silicide-based high-temperature protective coatings are still a hot spot for research, and in the future, we can continue to work on the composition and structural design of silicide coatings. In the composition design, it is necessary to ensure that the coating in the oxidation of the formation of a low oxygen diffusion rate of the flow dynamic glass phase oxide film, at the same time should ensure that the effective components of the coating in the oxidation process is not completely consumed. In the structural design, full consideration should be given to the interfacial bonding strength of the coating and the substrate, through the structural design, to realize the gradient distribution of the coefficient of thermal expansion between the coating and the substrate.
(2) Currently developed silicide-based high-temperature protective coatings are mainly for oxidizing environments below 1700°C. When the temperature rises above 1700°C, the SiO2 oxide film accelerates the volatilization and leads to the coating not being able to provide long-term protection. Therefore, how to improve the oxidation resistance of silicide-based coatings at high temperatures, especially in ultra-high-temperature environments, so that they can serve for a long time in a wide range of temperature domains is a problem that needs to be solved. Ultrahigh-temperature ceramic materials represented by ZrB2 have high melting point, low coefficient of thermal expansion and excellent thermochemical stability, and their oxidation at high temperature generates high melting point oxide ZrO2 with good adhesion and glassy B2O3 with low viscosity at high temperature and good fluidity, so the excellent antioxidant properties of ultrahigh-temperature ceramics can be combined with silicide-based coatings to prepare ultrahigh-temperature ceramic-modified silicides-based composite coatings, giving full play to the excellent antioxidant capabilities of the coatings and the excellent antioxidant properties of the ceramics. based composite coatings can be prepared by combining the excellent antioxidant properties of ultrahigh-temperature ceramics with silicide-based coatings to give full play to the synergistic effect of the components in the coatings under high-temperature conditions, which is expected to improve the long-term service capability of silicide-based coatings in ultrahigh-temperature environment.
(3) The high-temperature oxidation process of composite protective coatings with multi-component multilayer structure is relatively complex, and the process involves the mutual diffusion of elements such as B, Si, O and other elements in the multilayer coatings and molybdenum matrix, as well as the generation of a variety of compounds or intermediate phases, so clarifying the high-temperature oxidation mechanism of composite coatings and its protective mechanism is also a key issue in the optimization of coating composition and structure. Consideration can be given to combining thermodynamic calculations and oxidation kinetics to analyze the interactions between the elements in the oxidation process, the stability of the oxidation products and the evolution of the coating interface, to provide a theoretical and experimental basis for the optimization of the coating composition and structure design.
(4) At the present stage, the oxidation performance test for molybdenum and molybdenum alloy surface high-temperature protective coatings is mainly carried out in static air, while the actual service environment of molybdenum and molybdenum alloys is more complex (e.g., as the hot-end parts of the engine need to withstand the drastic change of temperature and the erosion of the high-temperature gas flow), and the evaluation of the anti-thermal shock performance and anti-ablative corrosion of the coatings should be considered. At the same time, the engine structural materials in the real service environment will also be subject to environmental deposits CMAS, molten salt impurities, water vapor and other corrosion factors, so it should also take into account the superposition of complex corrosion environmental factors. In addition, the influence of substrate shape and service environment factors should also be paid attention to in the selection of coating preparation technology and process parameters.
Reference: Surface Technology Volume 52, Issue 10, October 2023 Research Progress on High Temperature Protective Coatings on Molybdenum and Molybdenum Alloy Surfaces
Micron-sized spherical molybdenum powders and spherical molybdenum alloy powders prepared by Stardust Technology's RF plasma spheronization technology have the advantages of uniform particle size, good fluidity and smooth surface. These powders are widely used in 3D printing, electronic equipment, aerospace and other fields, and are particularly suitable for advanced manufacturing processes requiring high precision and high performance materials.
For the demand or customization of spherical powders of rare refractory metals, welcome to consult our professional manager Cathie Zheng +86 13318326187; we will be happy to answer your questions.


