Surface modification of titanium-tantalum alloy and its in vitro osteogenic bioactivity
Release time:
2025-07-23
Bone defects caused by congenital bone disease, trauma, and infections are common clinical conditions, which usually require the implantation of bone restorative materials for treatment[1] . Depending on the material, bone repair implant materials can be categorized into three types: metal, polymer and ceramic[2] , among which metal materials are preferred because of their good mechanical properties and relatively easy processing. Among the many biomedical metal materials, titanium and its alloys are favored because of their excellent mechanical properties, corrosion resistance, biocompatibility, ease of processing, and low price[3-4] . There are many successful cases of titanium and its alloys being used in the treatment of osteonecrosis, repair of bone defects, hip replacement, etc.[5-7] . Compared with Ti, tantalum (Ta) has better chemical stability and excellent biocompatibility [8], and thus has become a new material for bone repair in recent years. However, the scarcity of tantalum resources makes it expensive, and its high melting point makes it difficult to be processed, thus greatly limiting its popularization in biomedical applications. Instead, titanium and tantalum are melted and subsequently processed to make substable β-type TiTa alloys, which not only inherit the advantages of the two elements, but also have excellent plasticity and elastic modulus closer to that of human bone tissue, which make them potential candidates for hard tissue repair and replacement in the human body[9-11] . However, it is worth noting that titanium or tantalum metals have limited surface-mediated bone bioactivity, and it takes a long time for them to establish stable chemical bonding with the surrounding bone tissue after implantation into the organism. Therefore, in order to minimize the risk of implant failure due to displacement and loosening of the implant during the early stages of implantation, surface treatment of titanium or tantalum to enhance its bioactivity and thereby accelerate its integration into the bone tissue is essential. Constructing a hydroxyapatite (HA) coating on the implant surface with a bone-like composition is the most direct way to rapidly increase its osteogenic bioactivity. However, HA coatings produced by methods such as plasma spraying [12], biomimicry [13], and electrochemical deposition [14] are susceptible to delamination, cracking, and detachment after implantation into biological organisms due to the presence of thermal stresses in the coatings, or due to the low bonding strength to the substrate, thus affecting the clinical results. Therefore, it is increasingly important to destroy the original dense passivation film structure of biomedical metal surfaces by chemical or electrochemical means [15-16] and reconstruct the bioactive film as a transition layer, so as to realize the rapid deposition of calcium and phosphorus ions in the form of HA on the surface of the substrate under the physiological environment, and then establish a strong osseointegration between the implant and the hard bone tissues of the human body.
In this work, TiTa alloys were selected as the research object and surface modified by a simple one-step sodium hydroxide hydrothermal treatment or a two-step hydrothermal-precalcification treatment, and then evaluated the in vitro osteogenic bioactivity of the modified TiTa alloys with the help of simulated body fluid (SBF) immersion experiments, and analyzed the reasons for the enhancement of the osteogenic bioactivity in the light of the results of the experiment. We also analyzed the reasons for the enhancement of osteogenic bioactivity with the experimental results, hoping to provide valuable reference for the clinical application of this alloy.
1 Experimental materials and methods
1.1 Reagents
Sodium hydroxide (NaOH, AR), anhydrous ethanol (CH3CH2OH, AR), sodium chloride (NaCl, AR), sodium bicarbonate (NaHCO3, AR), potassium chloride (KCl, AR), potassium hydrogen phosphate trihydrate (K2HPO4-3H2O, AR), magnesium chloride hexahydrate (MgCl2-6H2O, AR), potassium chloride (KCl) and magnesium bicarbonate (MgCl2-6H2O) were used as reagents. MgCl2-6H2O, AR), calcium chloride (CaCl2, AR), sodium sulfate (Na2 SO4, AR), hydrochloric acid (36%-38%, mass fraction, the same below, HCl, AR), tris(hydroxymethyl)aminomethane (C4H11NO3, Tris, AR), and the above reagents were purchased from Shanghai Taitan Technology Co. The water used in the experiments was homemade deionized water.
1.2 Material preparation
Titanium-tantalum alloy (Ti:Ta=43:57, mass ratio) of 10 mm × 10 mm × 2 mm size was polished using 600# water-resistant sandpaper to remove the inert oxide layer on the surface. Afterwards, the alloys were ultrasonically washed in deionized water and anhydrous ethanol for 5 min to remove the surface oil. The washed TiTa alloy was transferred into a 100 mL polytetrafluoroethylene hydrothermal kettle containing 30 mL of NaOH solution (1 mol/L), and then sealed and reacted for 24 h at 70 ℃. At the end of the reaction, the TiTa alloy was removed and washed with deionized water for several times to remove the residual solution on the sample surface. One portion of the sample was dried at 50 ℃ and stored, while the other portion was subjected to pre-calcification.
The pre-calcification process: the NaOH-treated samples were dipped into saturated calcium chloride solution at 40 ℃ for 12 h, then washed with deionized water and transferred to K2HPO4-3H2O solution (1 mol/L) at 40 ℃ for 4 h. The samples were removed and washed with deionized water, then dried at 50 ℃.
1.3 Analytical characterization
The crystalline structure of the samples was characterized with the aid of an X'Pert Pro-type grazing incidence X-ray diffractometer (GIXRD) with a grazing incidence angle of 1.5 °, a Cu target, and Kα rays at a scanning angle of 10 °~75 ° and a scanning step rate of 2 ( ° ) /min. The samples were scanned with a Quanta 600F field emission scanning electron microscope (FESEM) and its ancillary equipment, and a Quanta 600F FESEM with its ancillary equipment. The samples were analyzed by Quanta 600F Field Emission Scanning Electron Microscope (FESEM) and its attached X-ray spectrometer (EDS, Oxford) for morphology and elemental composition. The chemical composition and elemental valence states of the samples were analyzed by a Kratos XSAM-800 X-ray photoelectron spectrometer with an Al target and Kα rays as the emission source. The static water contact angle of the samples was measured using a DropMeterTM A-200.
1.4 In vitro bioactivity evaluation
The in vitro osteogenic bioactivity of the samples obtained from the one-step hydrothermal modification and the two-step hydrothermal-precalcification treatments was evaluated using the SBF immersion assay. SBF was prepared as follows [17]: 8.035 g NaCl, 0.355 g NaHCO3, 0.225 g KCl, 0.231 g K2HPO4-3H2O, 0.311 g MgCl2-6H2O, 39 mL HCl (1 mol/L), 0.292 g CaCl2 , 0.072 g Na2 SO4 and 6.118 g Tris were dissolved in about 940 mL of deionized water, and then 1 mol/L HCl was added dropwise to adjust the pH value of the solution to 7.40 (37 ℃), and the solution was fixed to 1 L. The modified samples were put into 50 mL of SBF and incubated at 37 ℃, and then the samples were taken out and washed with deionized water and dried. After incubation for a predetermined period of time, the samples were removed and washed with deionized water, dried and used for subsequent characterization. The SBF should be replaced every 24 h during the incubation.
2 RESULTS AND ANALYSIS
2.1 Morphological analysis
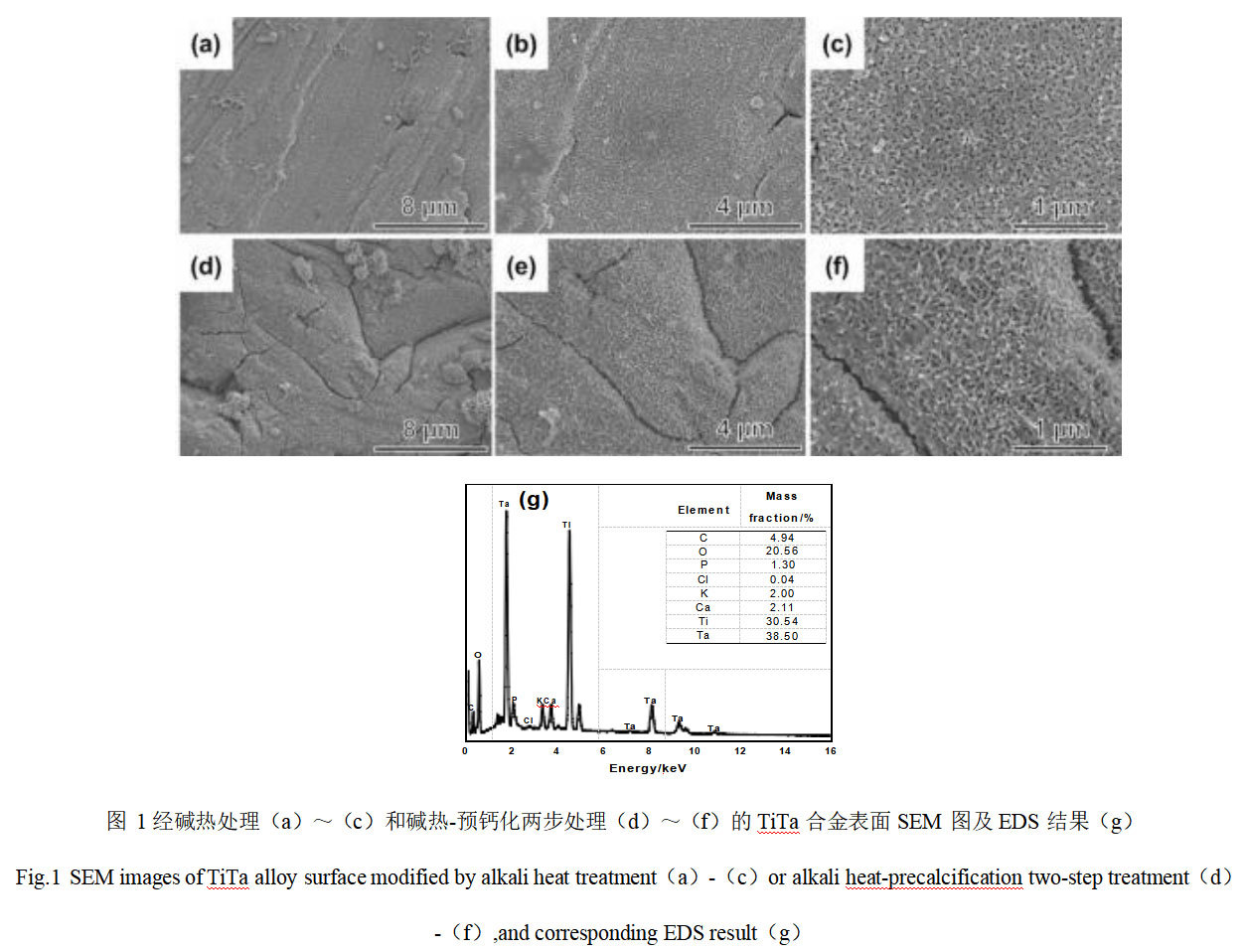
Fig. 1 shows the SEM and EDS analysis results of the surface of TiTa alloy after alkali heat treatment and alkali heat-precalcification. From Fig. 1(a) and (b), it can be seen that there is a continuous dense film on the surface of TiTa alloy after hydrothermal treatment in 1 mol/L NaOH solution for 24 h. High magnification micrographs show that the surface of TiTa alloy has been treated with alkali heat treatment and alkali heat-precalcification. As shown in the high magnification micrographs (Fig. 1(c)), the film layer is rough and assembled by many tiny nanowires, showing a reticulated porous structure. Figures 1(d)-(f) show the SEM images of the surface of TiTa alloys after two-step hydrothermal-precalcification treatment. Comparing with the one-step hydrothermal treatment, it can be seen that the pre-calcification treatment has basically no effect on the morphology of the samples. Figure 1(g) shows the EDS analysis results of TiTa alloy surface after two-step modification treatment. In addition to Ta, Ti, and O elements, Ca, C, P, and K elements are also detected, indicating that the subsequent pre-calcification treatment has successfully introduced Ca and P elements into the reticulated film layer generated by alkaline thermal modification.
2.2 Crystallographic analysis
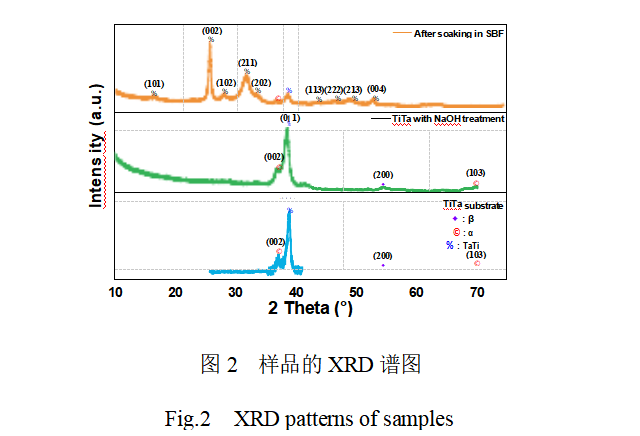
Figure 2 shows the XRD spectra of unmodified TiTa alloy, alkaline-heat modified TiTa alloy and alkaline-heat-pre-calcification modified TiTa alloy after SBF immersion culture. It can be seen that the unmodified treated TiTa alloy has a strong diffraction peak at 2θ=38.72 °, which corresponds to the (011) crystal plane of TiTa metal (JCPDSNo. 96-152-8104). In addition, several weak diffraction peaks were observed, which correspond to the α- and β-phases of titanium metal, respectively.The XRD spectra of TiTa alloys after alkaline thermal modification treatment showed little change compared to the pre-modification period, and no new crystalline diffraction peaks were observed. It has been reported in the literature[18-19] that the surface of titanium or tantalum metal after alkaline heat treatment will generate sodium titanate or sodium tantalate film, and the crystallization state of the film is greatly affected by the experimental hydrothermal treatment temperature, NaOH concentration, and other factors. In view of the low NaOH concentration and hydrothermal temperature used in this study, it is assumed that the film formed on the surface of TiTa alloys after alkaline heat treatment is amorphous sodium titanate (tantalum).
2.3 XPS analysis
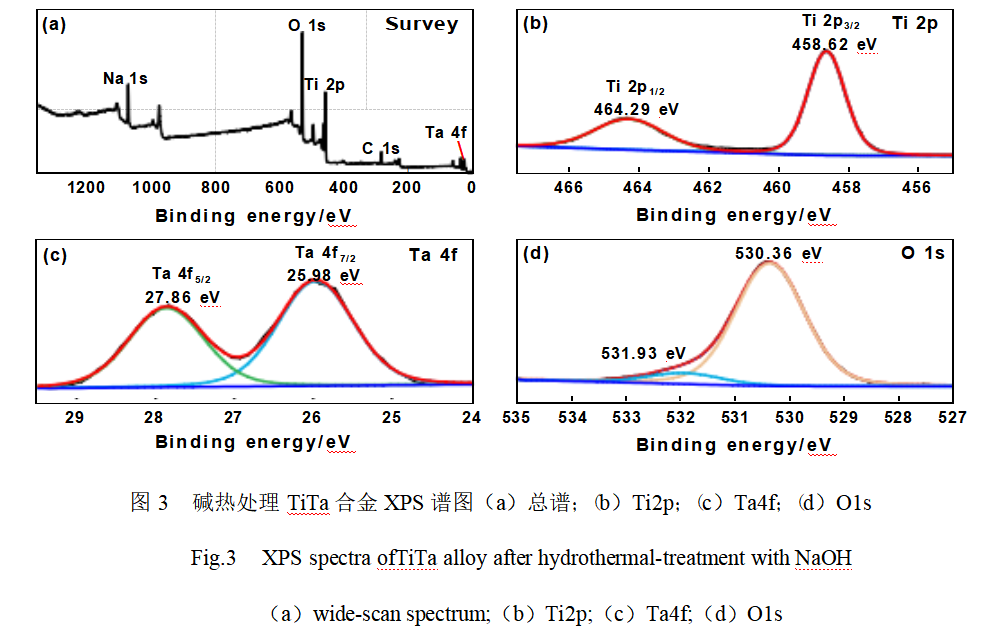
XPS was used to analyze the elemental composition and chemical valence state of the film layer on the surface of TiTa alloy after alkali heat modification treatment, as shown in Fig. 3. As shown in Fig. 3 (a), the characteristic signals of C1s, O1s, Na1s, Ti2p and Ta4f were detected in the full-scan spectra, among which the C1s signal at the binding energy of 284.8 eV was originated from the carbon contamination of the detector itself. Figure 3(b) shows the high-resolution XPS spectrum of Ti2p. Two signal peaks were observed at electron binding energies of 458.62 eV and 464.29 eV, which were attributed to Ti2p3/2 and Ti2p1/2, respectively, and the spin-orbit splitting value of 5.67 eV for both of them, confirming that Ti exists in the form of Ti4+ [20]. From the high-resolution XPS spectrum of Ta4f (Fig. 3(c)), the peaks of Ta4f7/2 and Ta4f5/2 are located at 25.98 eV and 27.86 eV, respectively, indicating that Ta exists in the form of Ta5+ [21]. The high-resolution spectrum of O1s in Fig. 3(d) can be fitted to two peaks located at 530.36 eV and 531.93 eV, which are attributed to Ti(Ta)-O and surface hydroxyl species, respectively [21-22].
2.4 In vitro osteogenic bioactivity
Osteogenic bioactivity is usually evaluated by the ability of the implant material to induce the deposition of ions such as Ca2+ and PO43- in body fluids as a bone-like hydroxyapatite layer on its surface under physiological environmental conditions. Therefore, in vitro immersion culture experiments of SBF were carried out on TiTa alloy samples modified in different ways. Fig. 4 shows the SEM images and EDS analysis results of the surface of alkali thermal modified TiTa alloys after different days of immersion culture in SBF. From Fig. 4(a) to (c), it can be seen that the surface morphology of alkaline-thermal modified TiTa alloys after 9 days of immersion in SBF is basically the same as that before immersion culture, which is still a reticulated structure composed of nanowires, and no nascent deposits are observed. When the immersion time was extended to the 18th day, as shown in Fig. 4(d)-(f), no obvious change in the surface morphology of TiTa alloy samples was observed. As shown in Fig. 4(g), no P element was detected except for the strong signal peaks of C, O, Ti and Ta and the weak signal peak of Ca. This confirms that there is no bone-like hydroxyapatite layer on the surface of alkali heat-modified TiTa alloys after soaking in SBF, which suggests that the osteoinductive properties are poor.
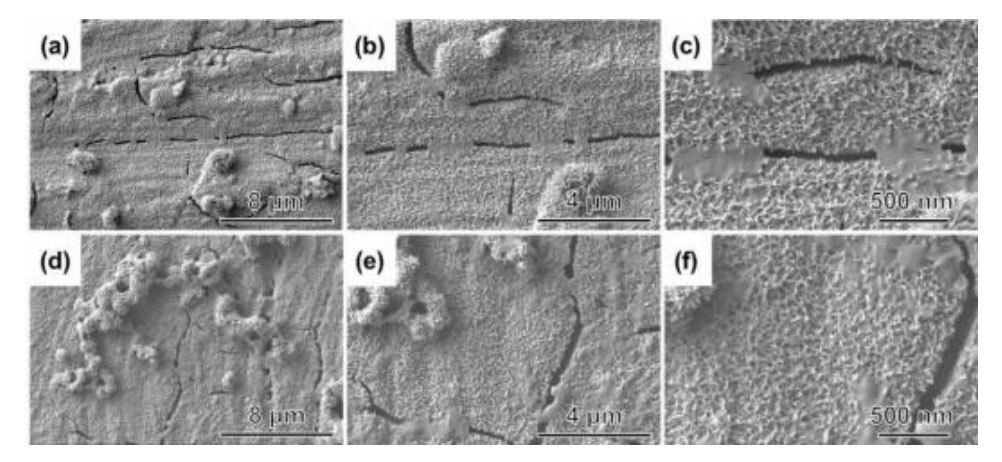
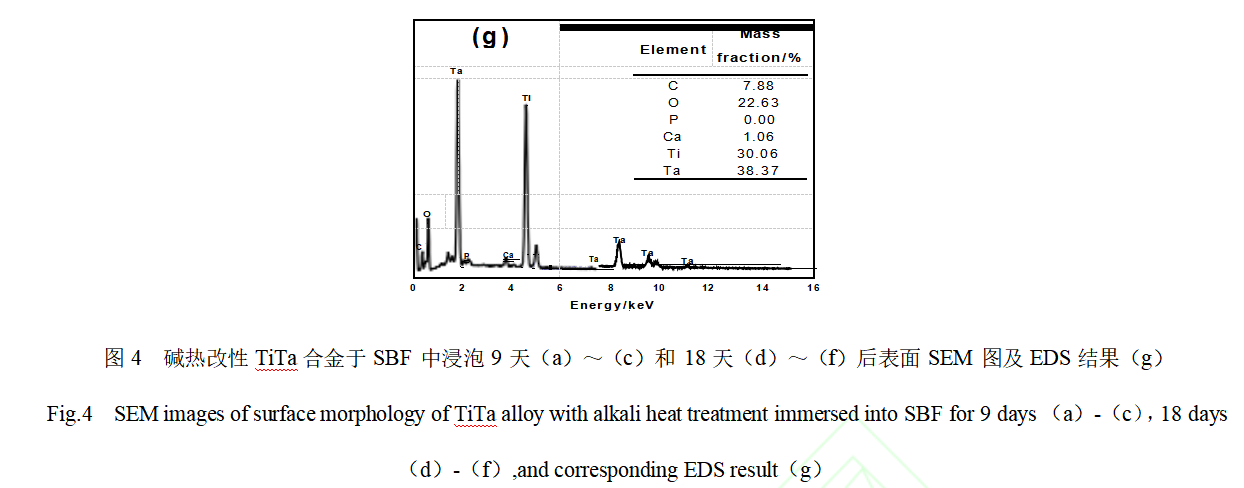
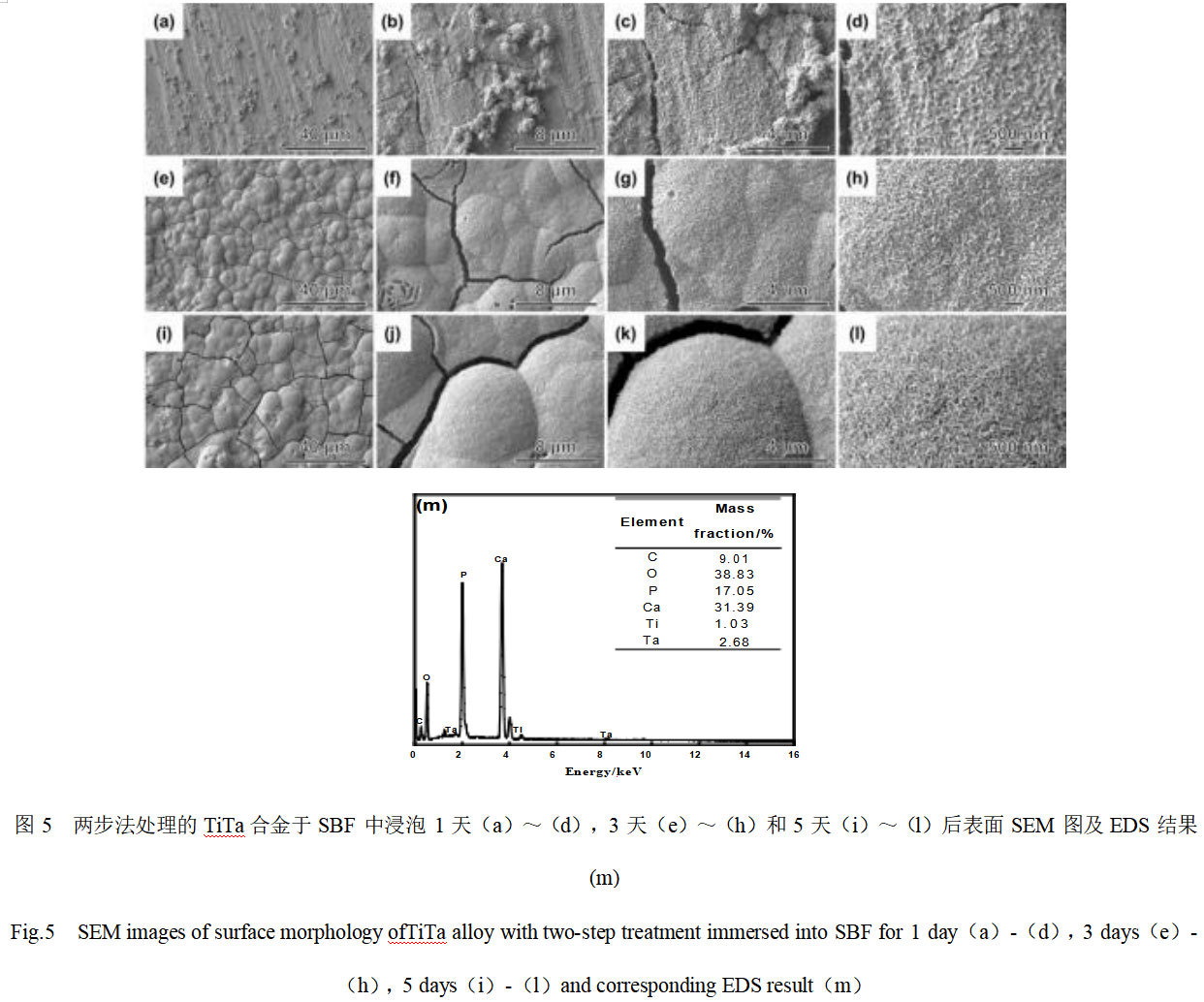
Fig. 5 shows the SEM and EDS analysis results of the surface of TiTa alloys treated by the two-step alkali heat-pre-calcification method after soaking and incubation in SBF for different days. From Fig. 5(a)-(c), it can be seen that the surface morphology of the TiTa alloys treated by the two-step modification method is basically the same as that before incubation after 1 day of immersion in SBF, but the high magnification electron microscope photographs (Fig. 5(d)) show that there are new-born deposits filling in the nanowire mesh structure on the surface. When the soaking time of SBF was extended to 3 days, the surface of the modified TiTa alloy was completely covered with newborn spherical clusters of several micrometers in diameter, as shown in Fig. 5(e)-(h). The magnified observation of these clusters reveals that they are porous mesh structures formed by nanosheets with a thickness of about 25 nm. In addition, cracks were observed in some areas of the film layer, indicating that the thickness of the nascent film-forming layer was large. When the SBF soaking time was further extended to 5 days, the clusters of spheres covering the surface of TiTa alloys became flatter and the cracks between the clusters became wider, indicating that the thickness of the formed layer increased with the increase of SBF soaking time. Fig. 5(m) shows the EDS analysis results of the surface layer of modified TiTa alloy after 5 days of incubation in SBF. The spectral results show that strong signal peaks of Ca, P, and O are detected in the layer, which is consistent with the expected results and indicates that these spherical clusters may be hydroxyapatite. The Ca/P mass ratio of the product is 1.84, which converts to an atomic ratio of 1.42, slightly lower than the theoretical Ca/P atomic ratio of 1.67 for HA (Ca5(PO4)3OH), suggesting that it may be a calcium-deficient apatite. In addition, the intensity of EDS signals originated from the Ti and Ta elements of the matrix was found to be significantly lower than that before incubation, which confirms that a thicker HA film layer was induced on the surface of the modified TiTa alloys, which is in agreement with the SEM results. In order to further confirm the phase composition of these spherical clusters, XRD analysis was carried out, and it was found that the strong diffraction peaks in the range of 2θ=25 °~33 ° matched well with the crystal faces of (002), (102), (211), and (202) of apatite (JCPDS No. 09-0432), which confirms that these spherical clusters of new material are apatite. The XRD spectrum (Fig. 2) also shows that the intensity of the diffraction peaks originating from the TiTa substrate is significantly reduced, which confirms that the apatite layer induced on the modified TiTa alloy surface is relatively thick. The results of in vitro SBF soaking experiments show that TiTa treated with alkali heat and pre-calcification has higher bioactivity than the NaOH hydrothermal modified samples, and a large amount of bone-like HA can be induced on the surface from the SBF in a shorter period of time, which demonstrates its potential application as a bone repair material.
2.5 Analysis of the reasons for the enhancement of biological activity
The reasons for the improvement of biological activity of TiTa alloys by NaOH hydrothermal modification supplemented with pre-calcification treatment are: firstly, after the hydrothermal treatment with sodium hydroxide, a porous reticulated amorphous sodium titanium (tantalum) oxide film layer assembled with nanowires was formed on the surface of TiTa alloys, which is usually favorable for inducing the formation of HA because of the porous, rough, and complex interfacial structure [23]. Secondly, the static water contact angles of TiTa alloys before and after alkaline thermal modification were (54.92±2.1)° and (11.16±2.4)°, respectively, which indicated that TiTa alloys were changed from hydrophilic to superhydrophilic after alkaline thermal modification, which was very favorable for the spreading of the SBF solution on the surface of TiTa alloys [24]. Furthermore, TiTa alloys contain Ti-OH and Ta-OH functional groups after alkali heat treatment, and when they are immersed in saturated CaCl2 solution, these functional groups will be deprotonated to form negatively charged Ti-O- and Ta-O- groups, which can adsorb Ca2+ through Coulombic gravity, thus making TiTa alloys positively charged. When immersed in K2HPO4 solution, the positively charged Ca2+ on the surface can quickly adsorb HPO42-, thus completing the nucleation of calcium-phosphorus compounds. It is well known that SBF is a supersaturated solution containing ions such as Ca2+ and PO43-. After the pre-calcification treatment, TiTa alloys, which already have initially nucleated calcium and phosphorus compounds on their surfaces, can be rapidly induced to precipitate calcium and phosphorus ions in SBF and gradually transform into the most thermodynamically stable calcium-phosphorus compounds, HA, after immersed in SBF [25-26].
3 Conclusion
(1) The TiTa alloy was hydrothermally treated with NaOH solution (1 mol/L) at 70 ℃ for 24 h. A porous and rough amorphous sodium titanate coating assembled with nanowires was constructed on the surface, and the static water contact angle was reduced from (54.92±2.1)° before the hydrothermal treatment to (11.16±2.4)°; and the alkali-thermally modified TiTa alloy was further pre-calcified. The further pre-calcification of alkali heat-modified TiTa alloys successfully introduced Ca and P elements into the surface reticulation layer, but the surface morphology was not significantly changed.
(2) The surface osteogenic bioactivity of the alkali heat-treated TiTa alloys was low, and the apatite layer could not be induced even after soaking in SBF for 18 days; whereas the surface of the alkali heat-pre-calcified TiTa alloys was covered by dense spherical hydroxyapatite coatings after soaking in SBF for only 3 days, which demonstrated excellent in vitro osteogenic bioactivity.
(3) The porous, rough and superhydrophilic surface structure formed by alkali heat treatment and the initial nuclei of calcium-phosphorus compounds introduced by further pre-calcification are the reasons for the improvement of the osteogenic activity of TiTa alloys, and the alkali heat-pre-calcification treatment can be widely used for the surface modification of other titanium alloys for biomedical applications.
Reference: Zou Yong, Liao Xiaogang, Yu Zi, Li Gang, Tian Tian. Surface modification treatment of titanium-tantalum alloy and its in vitro osteogenic bioactivity[J/OL]. Materials Engineering
Stardust Technology (Guangdong) Co., Ltd. focuses on the research, development and production of high-end spherical titanium-tantalum (Ti-Ta) alloy powders, providing customized products in various ratios, such as Ti-75Ta, Ti-50Ta, Ti-60Ta, and so on. Prepared by RF plasma spheronization technology, they are characterized by high purity (low oxygen content), high sphericity, no satellite balls, excellent fluidity and high vibration density. The microstructure is dominated by β-phase, and the fine particle size powder contains martensitic α-phase, and the hardness increases with decreasing particle size. The products are suitable for 3D printing (SLM molding densities >99%), orthopedic implants (elastic modulus close to that of human bone) and aerospace applications. For more details, please contact our professional staff, Cathie Zheng +86 13318326187.


