Study on oxidation behavior of NbW alloy at 600-900℃
Release time:
2025-07-22
With the rapid development of industry and the great improvement of people's living standards, the amount of civil and commercial electricity consumption has grown from kilowatts to megawatts. The traditional power generation model is not only difficult to meet the growing electricity consumption, but also brings great pollution to people's living environment. The development of nuclear power can not only solve the current problem of energy shortage, but also nuclear reactor power generation will not emit pollutants such as carbon dioxide, nitrogen oxides and sulfides caused by smoke into the air, reducing greenhouse gas emissions. At present, my country's nuclear power plants mainly rely on pressurized water reactors for power generation. The operating temperature of the reactor is about 350℃, and the thermal efficiency is only about 33%. With the development of the new generation of nuclear power plant technology, the reactor operating temperature is expected to be above 800℃ [1-4], and the thermal efficiency will be as high as 40%, which can effectively improve resource utilization. The refractory metal niobium and its alloys have a small thermal neutron absorption cross section, good corrosion resistance, and can maintain high strength and good processing performance at high temperatures [5-7]. They have attracted the attention of a large number of material science and technology workers [8-12] and can be used as structural materials for nuclear reactors and cladding materials for nuclear fuel. Niobium alloys are very sensitive to gaseous element oxygen. At low temperatures, they easily react with oxygen to form oxides on the surface, which deteriorates their performance and affects their use. This paper studies the oxidation performance of NbW alloys in low-oxygen environments in order to provide a basis for evaluating the application of NbW alloys in the nuclear industry.
1 Test materials and methods
The oxidation test specimens are NbW alloy tubes with a wall thickness of 0.7 mm, an outer diameter of 12.5 mm, and a length of 10 mm. The composition and original surface morphology of the alloy are shown in Table 1 and Figure 1, respectively. The surface of the specimen is the rolled surface after recrystallization annealing. The cut surfaces at both ends of the specimen are polished with 2000 sandpaper, ultrasonically cleaned with water and alcohol in turn, and then dried. Oxidation tests were carried out in a quartz tube multi-atmosphere reaction tube furnace. The test temperatures were 600 ℃, 700 ℃, 800 ℃ and 900 ℃, respectively. The oxidation time was 1h, 5h and 9h, respectively. The oxidation atmosphere was helium-oxygen (20×10-6), and the gas flow rate was 80mL/min. The morphology of the oxide film on the surface of the sample and the oxide composition were analyzed using a Zeiss evo10 scanning electron microscope from Germany and a D8 ADVANCE X-ray diffractometer from Germany's Bruker Company.
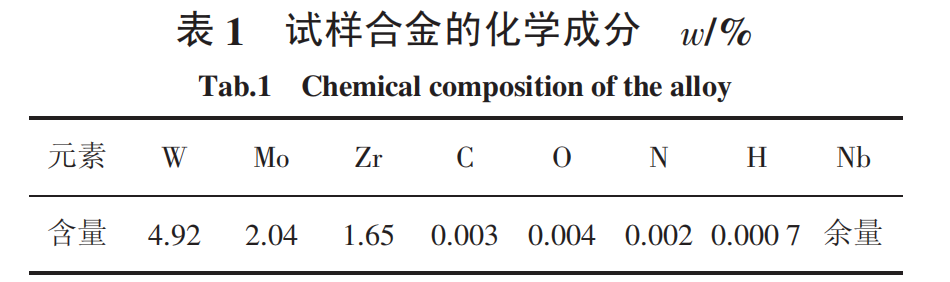
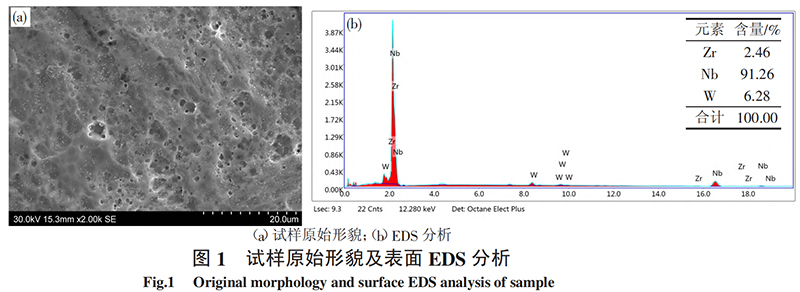
2 Test results and analysis
2.1 Oxidation kinetics
Figure 2 shows the oxidation kinetic curves of the alloy at 600 ℃, 700 ℃, 800 ℃ and 900 ℃. From the analysis of Figure 2, it can be seen that the oxidation weight gain of the alloy increases with the extension of oxidation time, and the change is very obvious with the increase of temperature. In the same time, the weight gain is most significant at 700℃, which shows that temperature has a great influence on the oxidation behavior of the alloy. The protectiveness of the oxide film produced at 700℃ is the worst. Therefore, the alloy should avoid long-term use below 700℃.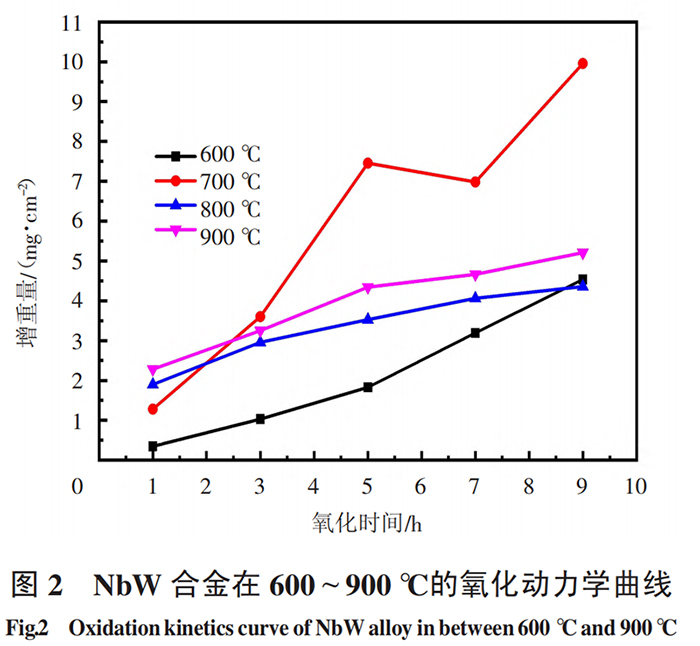
2.2 Macroscopic and microstructural changes of oxide film
Figure 3 shows the macroscopic morphology of the alloy after oxidation at 600℃, 700℃, 800℃ and 900℃ for 1h, 5h and 9h respectively. It can be seen from the figure that after the sample was heated at 600℃ for 1h, the surface color had changed, and the surface appeared blue with partial light yellow. When the oxidation time was extended to 9h, the surface color turned gray and black oxide powder began to appear; when the oxidation temperature was raised to 700℃, the oxidation began to intensify. After oxidation for 1h, the surface of the sample was gray with a small amount of black oxide. When the oxidation time reached 9h, the sample was severely oxidized and damaged; with the further increase of the oxidation temperature, at 800℃ and 900℃, the oxide film on the surface of the sample was dark gray and the oxide film was intact. From the above test results, it can be seen that the alloy has poor oxidation resistance at 600 ℃ and 700 ℃, and the alloy cannot be used for a long time, but it has excellent oxidation resistance at 800 ℃ and 900 ℃.

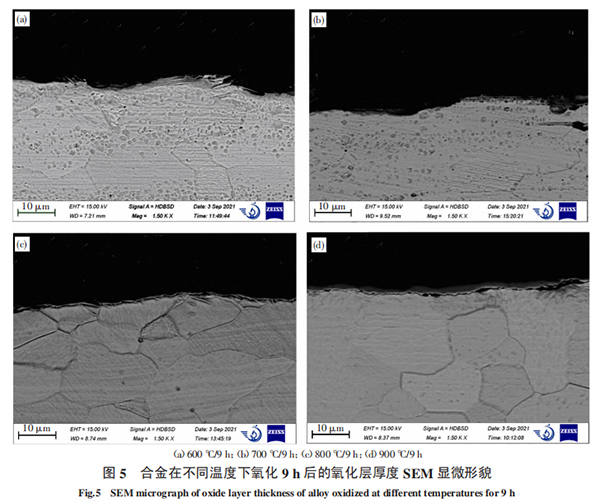
Figure 4 shows the microstructure of the alloy after oxidation at 600 ℃, 700 ℃, 800 ℃ and 900 ℃ for 1h, 5h and 9h, respectively, and Figure 5 shows the thickness of the oxide layer of the alloy after oxidation at 600 ℃, 700 ℃, 800 ℃ and 900 ℃ for 9h. It can be analyzed from Figure 4 that at 600 °C, an oxide film has been formed on the surface of the metal substrate and begins to nucleate to form a ridge distribution, presenting a honeycomb state, and white particles are generated on the surface of the oxide film. As the oxidation time increases, the white particles become more and more dense; when oxidized at 700 °C, the ridge morphology tends to be smooth, and the white particles gradually disappear; as the oxidation temperature increases to 800 °C and above, other particles are generated in the honeycomb and gradually grow, eventually making the oxide film dense and smooth. In addition, it can be seen from Figure 5 that when oxidized at 600 °C and 700 °C, no obvious oxide layer thickness is observed, the oxide layer is more obvious after oxidation at 800 °C, and the oxide layer begins to thicken after oxidation at 900 °C. This is because, when oxidized at 600 ℃ and 700 ℃, oxygen atoms diffuse into the metal matrix through chemical adsorption. Due to the existence of vacancies, gaps, dislocations and other crystal lattice defects in the metal matrix grain structure, the diffusion degree of oxygen atoms in the matrix is different. Therefore, the formed oxidation interface is not flat, but shows a ridge distribution. With the extension of heating time and the increase of oxidation temperature, the oxidation interface continues to advance inward, the lattice matching of the oxide changes, resulting in volume changes, which changes the local stress state inside the oxide film, and the oxide film becomes loose. Oxygen atoms continue to transmit to the inside through the holes, and the oxide film begins to fall off, making it difficult to form a protective film with a certain thickness. At ℃, due to the generation of new oxides, as the heating time increases, the amount of oxides formed gradually increases, gradually hindering the further diffusion of oxygen into the matrix. At this time, the formation of oxides causes the metal elements at the interface between the matrix and the oxide to become barren, causing the metal elements inside the matrix to gradually diffuse outward. The metal elements diffused to the interface continue to interact with the oxygen elements to form oxides. In this way, more and more nuclei are formed and connected into dense sheets, covering the metal surface. The oxide film isolates the oxygen atoms and has a protective effect. At the same time, the oxide layer gradually thickens.
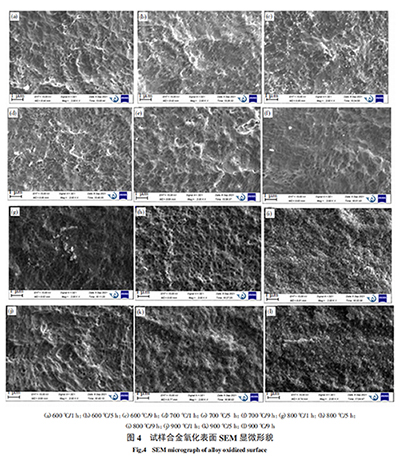
 2.3 Oxide phase changes in oxide film
2.3 Oxide phase changes in oxide film
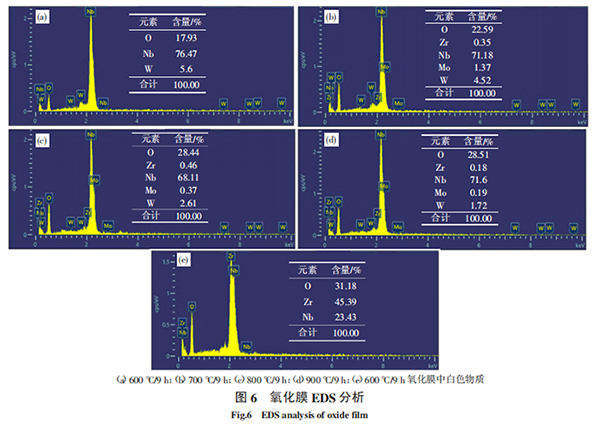
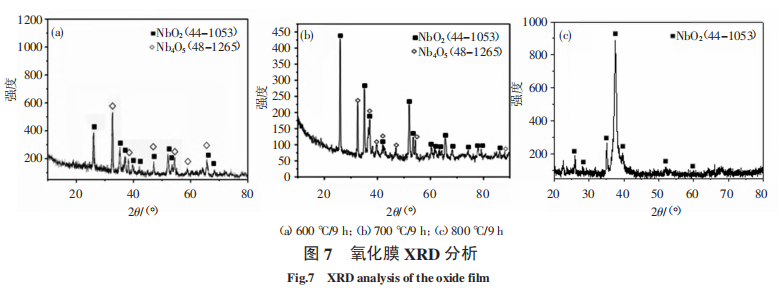
Figure 6 and Figure 7 are EDS and XRD analyses of the surface oxide film of the sample after oxidation at 600 ℃/9 h, 700 ℃/9 h, 800 ℃/9 h, and 900 ℃/9 h, respectively. As can be seen from Figure 6 (a) to Figure 6 (d), compared with Figure 1b, the surface oxygen content of the sample after oxidation at 600 °C is higher. With the increase of oxidation temperature, the surface oxygen content increases. After oxidation at 900 °C, the surface oxygen content does not increase significantly. In addition, as can be seen from Figure 6 (e), the white substance in the oxide film contains a high content of oxygen and Zr. From the XRD analysis in Figure 7, it can be seen that after oxidation at 600 °C, the surface oxides are NbO2 and Nb4O5. When oxidized at 700 °C, the surface oxides are mainly NbO2 and a small amount of Nb4O5; when the oxidation temperature reaches 800 °C, the surface oxide is NbO2. This indicates that part of the Nb in the oxide is replaced by Zr, and as the oxidation temperature increases, the oxide generated on the surface is changing, Nb4O5 is gradually decreasing, and NbO2 is gradually increasing. In the process of Nb4O5 decreasing and NbO2 increasing, the volume of the oxide will change, and the change in volume will generate stress in the oxide film, which will cause the oxide film to crack or partially detach during the stress release process, and the oxidation protection barrier will fail. As the volume effect increases, the oxide film falls off more and more seriously, unable to prevent the diffusion of oxygen atoms into the metal matrix, aggravating the oxidation of the alloy, and ultimately leading to metal oxidation weight loss and destruction; as the temperature rises to 800 ℃, the protective NbO2 oxide gradually increases, the oxide film gradually becomes dense, the anti-oxidation performance is enhanced, and the increase in oxygen content is no longer significant.
 3 Conclusion
3 Conclusion
(1) When niobium tungsten alloy is oxidized in a low-oxygen (20×10-6) environment at 600-700 ℃, the oxides formed on the surface during the oxidation process are mainly NbO2 and Nb4O5. With the increase of temperature, Nb4O5 decreases and NbO2 increases. The volume change caused by the change of oxides will generate stress inside the oxide film. The stress will cause the oxide film to crack during the release process, resulting in the failure of the protective property of the oxide film.
(2) When niobium tungsten alloy is oxidized in a low-oxygen (20×10-6) environment at 800-900 ℃, oxides mainly containing NbO2 are formed on the surface during the oxidation process. The oxide film is dense and protective, and the alloy is not easy to oxidize.
(3) The oxidation kinetic curve of niobium tungsten alloy shows that the alloy gains a lot of weight when oxidized at 700 ℃. The alloy is severely oxidized and is not suitable for long-term use at this temperature.
Paper citation information
China Tungsten Industry Vol. 36 No. 4 August 2021
The spherical Nb521 alloy powder produced by Stardust Technology is made by radio frequency plasma spheroidization process, with high purity and low oxygen, high sphericity, smooth surface, no satellite, uniform particle size distribution, excellent flow performance, high bulk density and vibration density. It is widely used in the manufacture of aerospace engines, weapon thrusters, rocket missile liquid bicomponent engines, nuclear reactors, submersibles, gas turbines, automobile engines, diesel engines, high temperature furnace heating belts, high temperature molds, high temperature fixtures, and high temperature crucibles.
http://en.stardusttech.cn/products_det/109.html
For more details, please contact Vicky Zhang at +86-13318326185


