Tightly Coupled Aerosolization Preparation of Thermal Sprayed NiCrAlY Alloy Powder
Release time:
2025-07-28
Aero-engines are developing in the direction of high power and high thrust, and the increase in inlet temperature of turbine engines leads to the difficulty of existing high temperature alloys to meet the requirements, and the application of thermal barrier coatings (TBCs) is the most effective way to increase the operating temperature of turbine engines [1 -3].
Thermal barrier coatings mostly adopt a two-layer structure, with a ZrO2-based ceramic layer on the surface, and a MCrAlY bonding layer between the ceramic layer and the substrate (M is the transition group metal Co, Ni or CoNi). Due to their excellent performance, MCrAlY alloys received wide attention in the mid-20th century.
In the 1970s and 1980s, the powdering and coating technology of MCrAlY was matured in Britain, the United States, Germany and Russia, and is still widely used and continuously improved [4]. Domestic research and development of MCrAlY started late, and there is an obvious gap with Europe and the United States and other countries. In recent years, China's aviation industry is booming, the market demand for high-performance aero-engines is huge, and the preparation and coating properties of MCrAlY alloy powder need to be improved urgently. There are many reports on MCrAlY coating performance research at home and abroad, but fewer reports on MCrAlY alloy powder preparation, and the preparation of high-performance powder is the first condition for obtaining an ideal coating. The Beijing General Research Institute of Mining and Metallurgy (BGRIMM) and the Institute of Metals of the Chinese Academy of Sciences (IMS) have conducted some experimental explorations on the preparation of MCrAlY alloy powder by aerosolization and the improvement of the alloy system [1,5]. In this paper, NiCrAlY alloy powder was prepared by tightly coupled aerosolization technology, and the powder morphology, bonding form, microstructure and elemental segregation were investigated, the relationship between solidification organization, particle size and cooling rate was analyzed, and the reasons for the generation and change of solidification organization, shrinkage cavities, and elemental segregation were discussed, with a view to providing experimental data for the production of high-quality thermal spraying with MCrAlY powder.
1 Experimental materials and methods
NiCrAlY alloy composition (mass fraction) of 25.0% Cr, 8.0% Al, 0.5% Y, 0.2% Si, the balance of Ni. Experiments using vacuum induction melting gas atomization (vacuuminertgasatomization, VIGA) process, the alloy powder preparation process route for the alloy raw material preparation (electrolysis Ni plate) The process route of alloy powder preparation is as follows: alloy raw material preparation (electrolytic Ni plate, Cr block, Al block, Si block and Y ingot) → vacuum induction melting → tightly coupled gas atomization → powder condensation → powder collection → grading → powder post-treatment. The raw materials are weighed and fabricated in the magnesium sand crucible of the vacuum induction furnace. Before the smelting furnace is energized, mechanical pumps and Roots pumps are first used to evacuate the melting chamber and atomizing cylinder, and the charge is melted into metal liquid in the medium frequency induction furnace, and atomized after controlling the temperature and other parameters of the metal liquid appropriately. Adopting self-designed tightly coupled gas atomization spray disk, the liquid guide tube is made of zirconium oxide, with an inner diameter of 8mm, the atomization medium is pure argon, the gas pressure is 3.5MPa, and the gas flow rate is more than 30m3-min-1. The temperature of the liquid metal atomization is from 1630 to 1670℃, and the liquid metal flows through the bottom of the middle packet and is atomized into molten droplets, which are condensed into powders inside the atomization cylinder, and the raw powders are graded into samples of various particle size intervals. The raw powder is graded and made into samples of various particle size intervals.
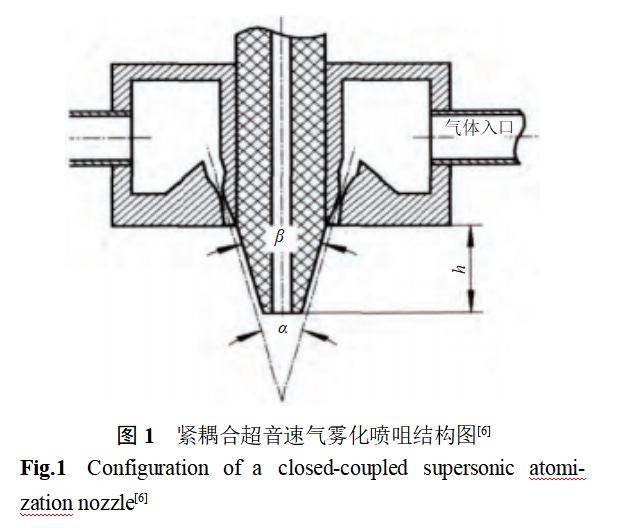
In the melting process using vacuum induction melting device greatly reduces the active elements Al, Y melting control difficulties, reduce the liquid steel oxygen absorption and inclusions in the formation of the medium frequency induction furnace electromagnetic stirring is conducive to the liquid steel in the melting period and atomization process of the element homogenization. The structure of the tightly coupled supersonic gas atomization nozzle is shown in Figure 1 [6], where α is the nozzle airflow angle, (°); β is the cone top angle of the metal guide tube, (°); h is the protruding height of the metal guide tube, mm. Atomization, the liquid steel flow out of the guide tube is tightly coupled with the high-speed atomization airflow, and the kinetic energy of the atomization airflow is converted into the surface energy of the liquid steel droplets to form a “V” shaped atomization process under the guide tube. The “V”-shaped gas reflux area under the liquid guide tube is conducive to melt film formation and primary crushing, and promotes the refinement of powder products. The combined application of tightly coupled gas atomization technology and vacuum induction melting process promotes atomized powder products with small particle size, good sphericity, precise composition, uniform element distribution, low oxygen and low inclusions.
Powder bulk density and fluidity were determined using Scott's funnel, particle size distribution was tested by sieve analysis, and chemical composition was tested using chemical titration. JSM-6400 scanningelectronmicroscope (SEM) was used to detect the powder morphology and analyze the elemental composition. The physical phase analysis of the powder was carried out using a 3kW rotating electrode X-ray diffraction (XRD) instrument of Bruker D8Discover, Germany, with a Cu target Kα ray source, λ=1.5406nm, excitation voltage 40kV and current 40mA. The sample powder was mechanically ground, polished and aqua regia corrosion to make a metallographic specimens, and the microstructure was observed in GX51 optical metallographic microscope.
2 Results and Discussion
2.1 Powder Physical Properties
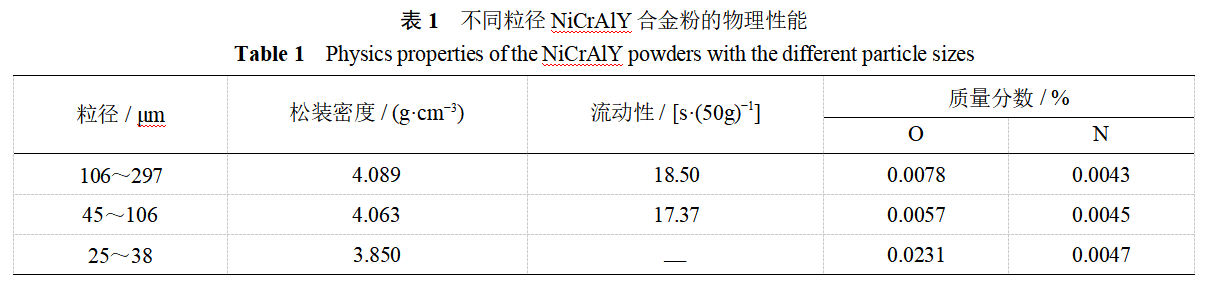
The fluidity, bulk density and trace element O, N content (mass fraction) of NiCrAlY alloy powder are shown in Table 1. The bulk density and fluidity of different particle size powders are obviously different, with the decrease of powder particle size, the bulk density decreases, the fluidity of coarse and medium-sized powders is similar and excellent, while the fluidity of fine-sized powders deteriorates sharply. The bulk density is not only related to the alloy material, but also closely related to the powder morphology and aggregation state. For large particle size powder, although the particle gap increases, but the phenomenon of adhesion bridges between particles to reduce the cumulative volume of powder gap in the unit volume of volume decreases, resulting in an increase in the bulk density; similarly, when the particle size is small, although the particle gap is less, but the particles are easy to form adhesion bridges, resulting in a decrease in the bulk density. When the powder has a high bulk density, the spray deposition rate is high. Powder fluidity is affected by powder morphology, particle surface state, particle size composition and other parameters. Similarly, when there is adhesion between the particles of the bridge phenomenon, both hinder the movement of powder, particle surface is also easy to adsorption of moisture and gas, so that the mobility deterioration. Fluidity is a key parameter affecting the smoothness of the spraying process of powder feeding, better fluidity is conducive to obtaining high-quality coating when spraying [1,6]. Powder oxygen content is the raw material oxygen content, smelting atomization process system vacuum and post-processing parameters control status and other factors together. Coarse particle powder due to the long condensation time, more oxygen absorption from the environment, fine particle powder due to the large total surface area of the particles, the total oxygen content is large. The oxygen content of different particle size powder in Table 1 also shows this law, the oxygen content of fine particle size powder is the largest value, followed by coarse particle size, and the lowest in the middle particle size. Table 1 can also be seen, regardless of the powder thickness, the nitrogen content is almost the same, more stable, which may be related to the nitrogen is fused to the solid solution rather than enriched in the particle surface. O, N and other impurity elements in the powder, usually in the form of MxOy or AluNv, thermal spraying will seriously damage the coating bonding strength, life, etc., so the bonding layer alloy powder to strictly control the O, N content. This paper does not use nitrogen and choose high-purity argon as the atomization medium, from the point of view of the impurity content of the powder, to achieve the desired purpose.
2.2 Powder particle size distribution
The particle size distribution of alloy powder is affected by the atomization process. The tightly coupled gas atomization process can be divided into three stages: primary crushing, secondary crushing and cooling solidification [7]. After completing the primary crushing in the gas reflux zone, the columnar steel stream follows the Weber number criterion We = ρ-v2-d-σ-1, where ρ is the density of the atomized gas, v is the relative velocity between the gas and the molten droplet, d is the diameter of the molten droplet, and σ is the surface tension of the molten droplet. When the Weber number of the droplet reaches a critical value, the secondary fragmentation may occur, and with the Weber number from small to large, the secondary fragmentation of the droplet is manifested as “dumbbell crushing”, “bag crushing”, "extended crushing “Dumbbell type crushing”, “bag type crushing”, “extended crushing”, “explosive crushing” and other modes, the final powder size varies greatly.
Figure 2 shows the interval particle size distribution and cumulative particle size distribution curve of NiCrAlY alloy powder. It can be seen that 85% of the powder particle size is less than 150 μm. 41% of the powder with particle size below 50 μm. The average particle size of the powder D50, expressed as the particle size corresponding to 50% cumulative volume fraction, is about 58 μm; similarly, D84 is 124 μm. The width of the powder size distribution δ = D84/D50, which can be calculated as δ = 2.138, indicating that the alloy powder has a wide particle size interval and is not uniformly distributed. Thermal barrier coating bonding substrate materials need to be selected according to the spraying process, ceramic powder parameters, application areas and powder comprehensive parameters of a certain particle size interval of the powder, its particle size distribution and atomization of air pressure, the inner diameter of the liquid guide tube, the degree of superheat of the liquid steel and other process parameters are closely related to the atomization parameters can be adjusted to change the particle size distribution of the received powder.
2.3 Powder morphology
Figure 3 shows the morphology of NiCrAlY alloy powders with different particle sizes. From Figure 3, it can be seen that regardless of particle size, the powder is mainly composed of spherical or nearly spherical particles, which contain a small number of shaped particles. The finer the particle size of the alloy powder, the better the sphericity of the particles and the smoother the surface. As the particle size increases, the sphericity of the powder deteriorates, the morphology is more similar to the tear-drop shape, and the surface of the particles has more folds and satellite particles, and the smoothness deteriorates.
From Fig. 3(a) and Fig. 3(b), it can be seen that the fine powder (micron-sized) is mostly in the single particle state, and with a slight increase in particle size, some of the powders are adhered. This is because the micron-sized particles are cooled quickly and solidified completely before collision, so they are not easy to bond with each other during flight cooling. Adhesive particles are mostly “wrapped” adhesion and ‘gourd’ adhesion, “wrapped” adhesion is by the particles within the first solidification, and then another droplet impact on the particles, the melt droplet extension and condensation. The “encapsulated” adhesion is formed by the encapsulated particles solidifying first, and then another droplet impacts on the particles, and the droplet extends and condenses; the “gourd” adhesion is formed by the collision of two solidified particles in which at least one of the two particles is not fully solidified, and the two are locally adhered to. These two types of adhesions are more likely to occur when there is a small difference in particle diameter [8-9]. Tightly coupled gas atomization “V” type gas reflux zone is small, but the reflux zone occurs in the liquid steel column → liquid film → melt droplets of the first and second broken, the flow field structure is very complex [7], greatly exacerbated the different sizes of molten droplets in the condensation of the chances of mutual adhesion. From Figure 3 (c) and Figure 3 (d) can be seen, with the particle size becomes larger, with satellite particles of the powder increased significantly, and many large powders even have more than one satellite particles, at this time the adhesion mode is mainly “raised” adhesion. It is generally believed that the “raised” adhesion is the rapid solidification of small particles and the surface is still not fully solidified large particles collision and bonded together.
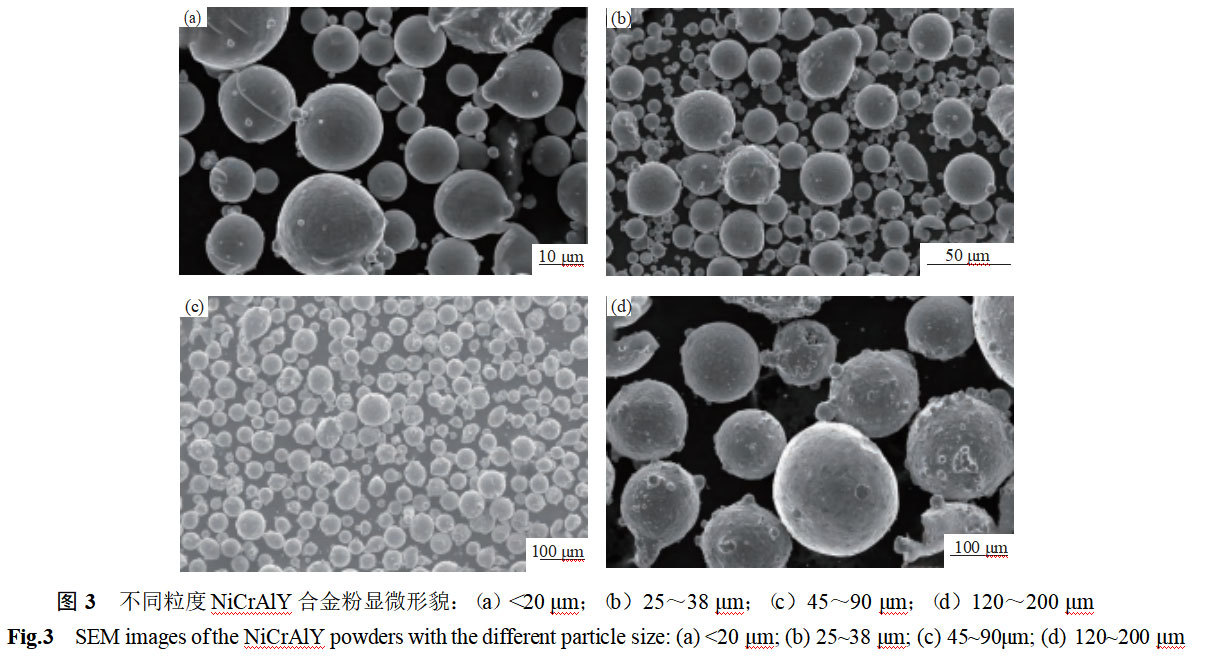
It is generally accepted that adherent powders are closely related to aerosolization parameters. The structure of the atomizing spray disk, air pressure, air temperature, gas flow rate, the size of the gas reflux field in the atomizing cylinder, exhaust rate, etc. are all influencing factors. Combined with the data in Table 1 can be seen, although the fine particle powder has a good degree of sphericity, particle surface smooth, but the powder mobility is poor, low bulk density. Although the coarse particle powder has poor sphericity, serious wrinkles and satellite particles on the particle surface, but the powder has good fluidity and high bulk density. This also shows again that the loose loading size and mobility are not only related to the powder morphology and surface, but also affected by parameters such as the particle size distribution and aggregation state of the powder.
2.4 Powder microstructure
Figure 4 shows the microstructure of NiCrAlY alloy powder particles with different particle sizes in section. The internal organization of the powder reflects both the solidification state of the alloy and the crystallization and growth status of the alloy during the solidification process. As can be seen from Fig. 4, the powder particles have two typical organizations of cytosolic and dendritic crystals, and the particles of different sizes are a mixture of cytosolic + dendritic crystals.
As can be seen in Fig. 4(d), the powder with a particle size less than 30 μm is mainly composed of cytocrystalline organization, but a small amount of dendritic organization has already appeared, and a small number of solidification shrinkage holes can be observed in the cross-section of the particles. With the increase of powder particle size, as in Fig. 4(b) and Fig. 4(c), the solidification organization consists of cytosolic crystals + dendritic crystals, the cytosolic crystals are radial growth, compared with Fig. 4(d), the proportion of dendritic crystals increased significantly, and the dendritic crystals tend to grow to a complete size, and the grains are more coarser, and the particles can be seen dispersed in a number of solidification shrinkage holes. As the powder particle size continues to increase, as seen in Figure 4(a), the powder microstructure is dominated by dendritic crystals, some dendritic crystals also appear secondary crystal organization, and cytosine crystals exist only in the local area. Comparison of Fig. 4(b) reveals that the number of solidification holes appearing within the particles is much higher, and the size increases significantly.
It can also be seen from Fig. 4 that, regardless of the particle size of the powder, the cytosolic crystalline organization is predominant at the edge of the cross-section, and the dendrites mainly appear near the middle of the particle cross-section, while the grain sizes of the cytosolic and dendritic crystals inside the powder are extremely inhomogeneous. The cooling rate of the molten droplets is the key parameter affecting the internal organization of the powder. The solidification of metallic molten droplets is a high-temperature, high-speed process involving the transition from liquid melt to solid, and direct measurement of the droplet condensation rate is quite difficult and is usually derived by indirect methods. The relationship between the secondary dendrite arm spacing and the cooling rate is λ=b-T-n, where λ is the secondary dendrite arm spacing, μm; T is the cooling rate, K-s-1; b is the modification constant, μm-(K-s-1)n; and n takes the value of 1/3 to 1/2 [10]. Adopting the “intercept method” [11], six to eight cell or dendrite spacings were randomly measured in Fig. 4, and the average of the measured values was taken as the crystal spacing, with b = 50 μm-(K-s-1)n and n = 0.33, to derive the particle cooling velocity. The data on grain spacing and cold velocity for different sized particles are shown in Table 2.
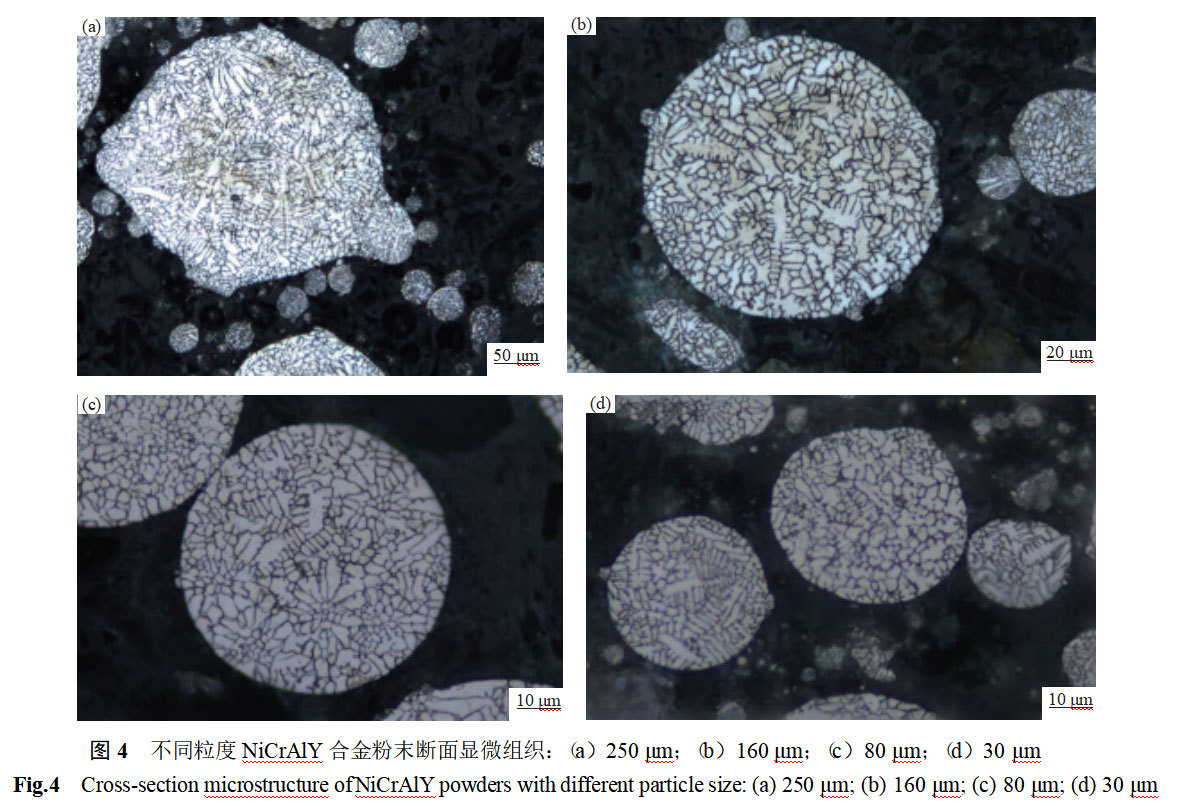
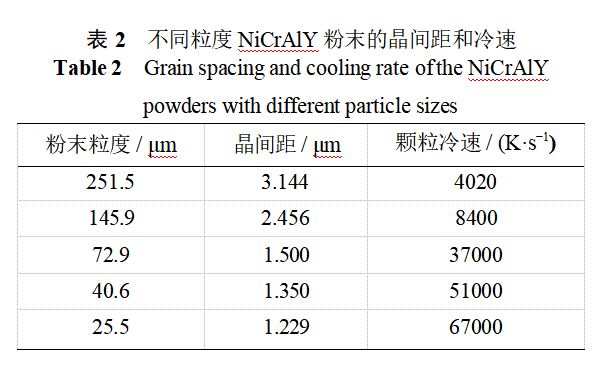
NiCrAlY alloy main elements Ni, Cr, Al melting point, atomic radius, density difference is obvious, the molten droplet condensation process tends to occur selective solidification, the formation of dendritic crystal organization, so the small particle size powder also appeared within the obvious dendritic organization. With the increase of the molten drop, the cooling rate decreases, large particle size powder tends to form dendritic crystal organization. However, the heat exchange between different parts of the large droplet and the environment is more complex, and the situation is different. Therefore, the large particle size powder in Figure 4 is a mixture of refined cytosolic and dendritic crystals, and even secondary dendritic crystals.
The surface finish of powders with different particle sizes is also affected by the solidification cooling rate. As shown in Figure 3, small particle size powder condensation rate is high, so that the crystallization process within the molten droplet is inhibited, and the surface of the molten droplet is only slightly contracted after condensation, so the surface finish of the powder is smooth; large particle size powder has a low cooling rate and a long condensation time, and it is easy to form the difference between the solidification contraction in the condensation, so that the surface finish of the large particle size powder is poorer [12].
Figure 5 shows the scanning electron microscope backscattered (SEM-BSE) morphology of NiCrAlY powder (45-106 μm) section. As can be seen from Fig. 5(a), the organization is overall uniform and dense, and the number of powders with shrinkage holes or hollow defects accounts for about 8% to 10%. Observation of individual enlarged particles revealed [12] that the internal structure of powders with cross-sectional diameters <50 μm was dense and no obvious metallurgical defects were observed, as shown in Fig. 5(b). Irregular condensation shrinkage holes appeared within some of the powders with cross-sectional diameters >50 μm, and even multiple shrinkage holes appeared within the large-size particles, as shown in Fig. 5(c). This is due to the fast cooling rate and large subcooling degree of the small-sized droplets during the condensation process, which promotes a large number of internal nucleation and inhibits grain growth by contacting each other between grains. Large-size droplets have a slow cooling rate and long solidification time, which makes it easy to form a dendritic crystal organization. Solidification shrinkage is formed due to the condensation contraction of the molten droplets, and when multiple dendrites are formed within the large-size particles, the degree of condensation contraction is greater, and multiple shrinkage holes are easily formed [13].
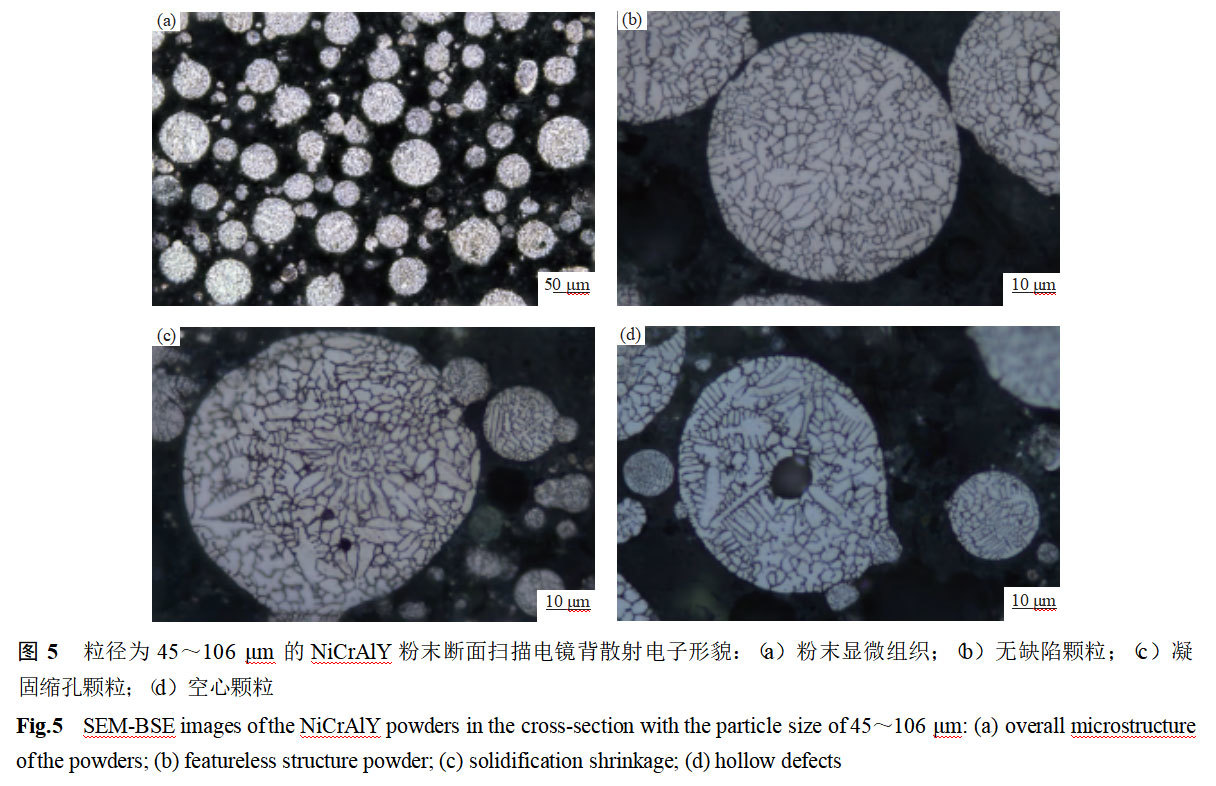
Hollow defects were also observed within particles with cross-sectional diameters >50 μm, and the hollows mainly appeared in the center part of the particles, as shown in Fig. 5(d). During tightly coupled atomization, the liquid column fragmentation may occur either as primary or secondary fragmentation due to the Weber coefficient (We). Studies have shown that when 12 <We <50, the secondary breakage is prone to “Bag” breakage mode. With the liquid film cold, the surface tension of the liquid steel rises rapidly, preventing the liquid film to continue to break, and may be atomized gas package, the droplets will form hollow defects after condensation. When the powder particle size is large, first of all, it is easy to meet the conditions of the Weber coefficient of the occurrence of “pocket crushing”, and secondly, the particles are generated by the spreading area of the liquid film condensation is larger, and increase the chances of wrapping bubbles to form the occurrence of hollow powder [14].
Solidification shrinkage holes and hollow defects within the alloy powder is caused by the alloy composition, preparation process and solidification mechanism, difficult to completely eliminate. When preparing the thermal barrier coating, if the shrinkage holes or hollow centers in the bonded substrate powder cannot be eliminated, the wrapped gas cannot escape, which has an impact on the coating bond strength, porosity and other indicators. Cooling rate is the key factor affecting the powder microstructure, the formation of crystal organization state, solidification shrinkage and hollow defects are affected by its role, and the cooling rate is affected by the structure of the atomizer and atomization process parameters.
2.5 Polarization of elements inside the powder
As the powder locks the compositional polarization within individual particles, it solves the macro-polarization of traditional metallurgical products, but the enrichment and depletion of elements inside the powder particles, and the polarization inside and outside the crystal cell also affect the stability of the powder properties.
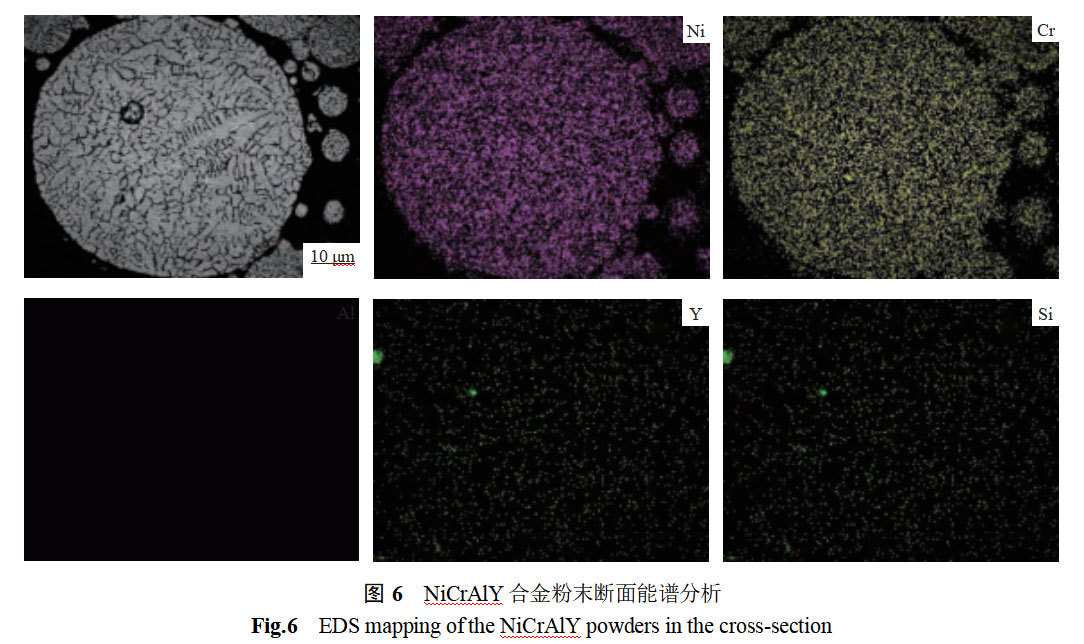
Figure 6 shows the energydispersersespectroscope (EDS) surface scanning analysis of the NiCrAlY alloy powder section. From Fig. 6, it can be seen that the major elements Ni, Cr and Al are uniformly distributed without obvious enrichment and depletion, while the microalloying elements Y and Si are unevenly distributed. The uniform distribution of major elements is due to the fact that the molten droplet atomization process is at a cooling rate of 103-104 K-s-1, and the elements inside the powder are not able to gather in time before they have solidified rapidly.
In order to further explore the elemental distribution of the powder internal grain cells, scanning electron microscopy and energy spectroscopy were utilized for the elemental determination of NiCrAlY alloy powders, and the measurement positions are shown in Fig. 7(a), where the face scanning was carried out on area A to detect the elemental distribution of intra-granular point B, grain boundary point C, and inter-granular point D. The elemental content of the powder was analyzed by scanning electron microscopy and energy spectroscopy. Fig. 7(b) and Fig. 7(c) show the energy spectrum analysis of intragranular point B and intergranular point C. The data of the content of each element are shown in Table 3. As can be seen from Table 3, compared with the mean value of the face scan of area A, the content of Si and Y at intergranular point C is on the high side, and the content of Al is on the low side, while the intragranular point B behaves in the opposite way. The Cr and Ni contents are basically the same at each test point, indicating the existence of slight elemental segregation at grain boundaries and within the grain [15-16]. Studies have shown [2] that Si and Y have low solid solubility in NiCrAlY alloys, and elemental segregation may occur when the elemental content of the metal liquid is high and the molten droplets condense at a low speed.Si can reduce the oxidation rate of the thermal barrier coatings and improve the adhesion strength of the oxide film and the high temperature antioxidant properties.Y can refine the grains, and improve the bonding of the oxide film of the thermal barrier coatings with the substrate.The occurrence of enriched segregation of Si and Y at grain boundaries can play a role of The enriched polarization of Si and Y at grain boundaries can play a role in hindering the movement of grain boundaries and improving the bonding strength of the thermal barrier coating.
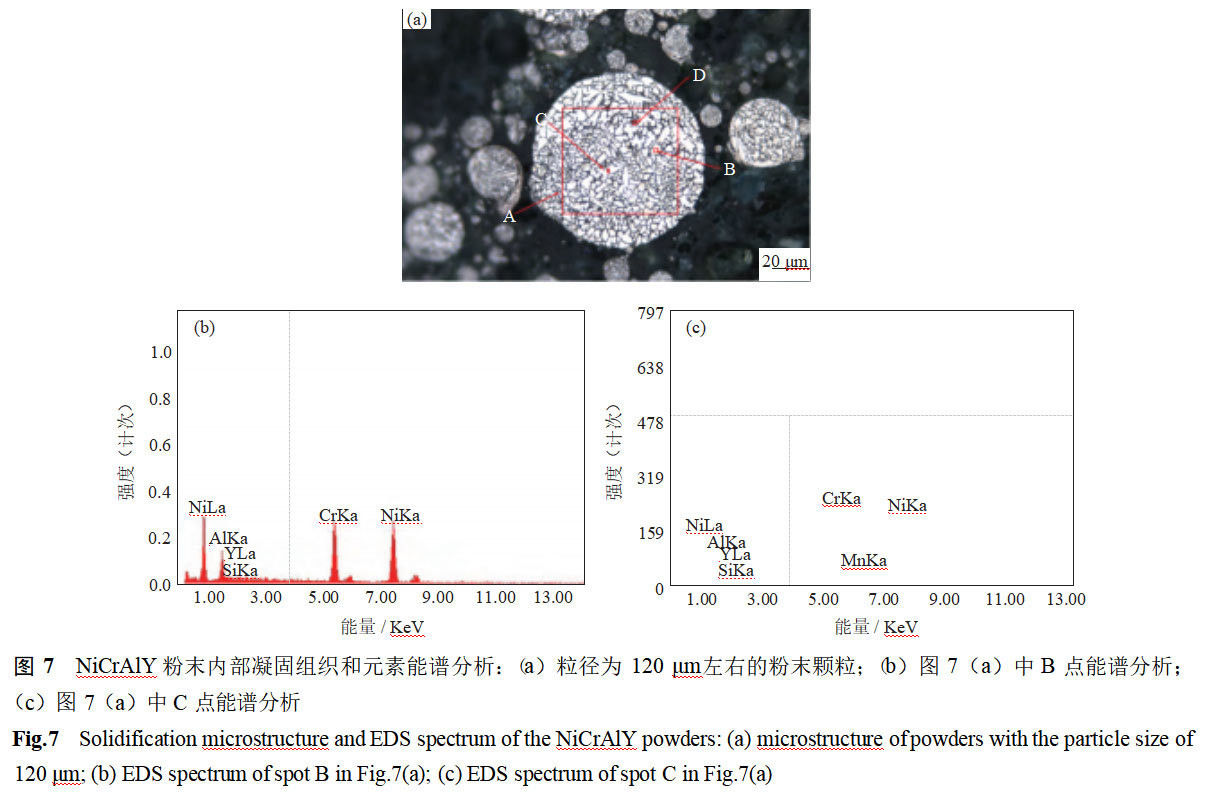
The data in Fig. 6, Fig. 7 and Table 3 show that the main elements of NiCrAlY alloy powder are uniformly distributed, while the microalloying elements Si and Y are unevenly distributed, and there is segregation. It is difficult to suppress such segregation. Small particle size particles can also rely on high solidification cold speed to inhibit the diffusion of alloying elements, weakening the segregation. For large-size powders with lower cooling speed, the measures are much less.
2.6 Phase structure of powders
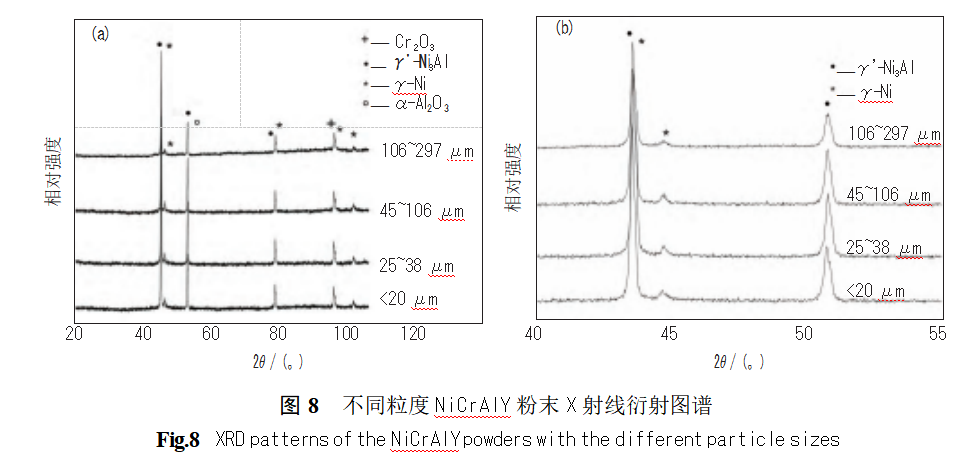
Figure 8 shows the X-ray diffraction patterns of NiCrAlY powders with different particle sizes. As shown in the figure, the phase composition of different particle size powders is basically the same, mainly composed of γ'-Ni3Al, γ-Ni, α-Al2O3, and Cr2O3 phases to form γ'+γ phase structure. Due to the different cooling speeds, the phase compositions and crystal structures of the powders with different particle sizes may differ [17]. The intensity and area of the γ-Ni (2θ=45°) diffraction peaks in the diffraction curves were gradually enhanced with decreasing powder particle size, indicating a significant increase in its content [18]. As shown in Fig. 8(b), the peak position of γ'-Ni3Al (near 2θ=52°) was shifted and tilted to the small angle direction with decreasing powder particle size, which was caused by the effect of supersaturated solid solution formation by rapid solidification. NiCrAlY powders with a particle size <20 μm have the highest cooling rate, the greatest degree of offset occurring, and the most severe lattice distortion, reflecting the fact that the higher the solidification rate, the higher the saturation of solute atoms in the γ'-Ni3Al solid solution [19-20].
3 Conclusions
(1) NiCrAlY alloy powders were prepared by tightly coupled aerosolization technique, and most of the powders (about 85%) had a particle size of less than 150 μm, with predominantly spherical and near-spherical particles. With the increase of particle size, the sphericity deteriorates, and the phenomenon of adhesion and satellite particles is serious.
(2) The microstructure of the powder is mainly composed of dendrites and cellular crystals, and the solidification cooling rate of the molten droplets with a diameter of 25-250 μm is 4000-67000 K-s-1.
(3) The internal structure of small particle size powder (particle size <50μm) is dense, and a small amount of large particle size (particle size >50μm) powder has solidification shrinkage holes and hollows, and there are elemental Si and Y segregation at the grain boundaries of the powder particles and inside the grain.
(4) The composition of the powder phase is mainly γ'-Ni3Al and γ-Ni, and the degree of lattice distortion is aggravated with the smaller particle size.
Spherical NiCrAlY alloy powder is a high-temperature protective coating material prepared by argon atomization or plasma spheronization process, with excellent sphericity, high fluidity and uniform elemental distribution (e.g., Cr, Al, Y), which mainly consists of γ/γ' or β phases. Its properties include excellent resistance to high-temperature oxidation (rapid formation of dense Al₂O₃ film to retard Al depletion) and corrosion resistance, but poor electrochemical corrosion resistance. Widely used in the repair of aero-engine hot end parts, gas turbine blade protection and anti-corrosion coatings for biomass fuel boilers, the characteristics include low porosity, high bonding strength and can be further optimized for high temperature performance by doping with elements such as Nb/Hf/Ta. For more powder inquiries, please contact our professional staff: Cathie Zheng +86 13318326187.

News

