Analysis of factors affecting the oxygen reduction performance of tantalum powder by the oxygen reduction process
Release time:
2025-07-21
The enhancement of the charging and discharging performance of tantalum capacitors and the preparation of highly reliable tantalum electrolytic capacitors are the main areas of development and competition in the current industry, and tantalum powder, as a raw material for the production of capacitors, is widely used in aerospace and communication equipment and other fields [1-5]. In recent years, with the progress of ceramic capacitors, aluminum capacitors production technology, some of the use of tantalum capacitors in the field suffered a pinch, in order to cope with the new challenges, tantalum powder is required to be able to have a large specific capacitance at high voltage, high breakdown voltage, small leakage current and low loss, to effectively meet the market demand. The quality of tantalum powder directly affects the performance of electrolytic capacitors, including leakage current, loss, specific capacitance, shrinkage and so on. The particle shape of tantalum powder, the cohesive state and the state of the surface oxide film are among the technical keys. Tantalum powder is usually made using the sodium reduction potassium fluorotantalate process, and the initial tantalum powder is obtained after reduction and acid washing, to be vacuum heat-treated to meet the requirements for use in making electrolytic capacitors. However, tantalum metal has a great affinity for oxygen, tantalum powder made after the above process, the formation of non-equilibrium solid-solution oxygen inside the particles, as well as by the surface activation of oxygen [6], which is very unfavorable for the production of electrolytic capacitors, which will cause poor capacitor voltage resistance, the reliability of the dielectric film decreases, the leakage current is large, so that the capacitor's performance is reduced, and cause tantalum wire is brittle, and the anode to make the capacitor has poor life characteristics, so Oxygen reduction of tantalum powder must be carried out.
Magnesium reduction process was first developed by Stantec tantalum powder preparation technology, is above 800 ℃, the use of gaseous magnesium reduction of tantalum oxide to get micro-fine powder [7]. The gaseous magnesium reduction of tantalum oxide was used to produce microfine tantalum powder by Stantec, Germany [8], The magnesium reduction of tantalum oxide process is usually carried out by liquid-solid sintering and gas-solid sintering. Existing processes discussed tantalum powder, niobium powder oxygen reduction related process research, experiments using tantalum powder magnesium treatment oxygen reduction process [9], comparative analysis of different magnesium doping, reaction temperature, holding time, oxygen reduction out of the furnace, after pickling of the physical and chemical properties, focusing on the analysis of the difference between the electrical properties, in order to provide a basis for effective improvement of the quality of tantalum powder.
1 Experiment
1.1 Main equipment and raw materials
1.1.1 Experimental equipment
The experimental equipment is a well oxygen reduction furnace, pickling tank, oven, analytical equipment for LECOCS-436 oxygen and nitrogen analyzer, GE340 spectrometer, WLP202 average particle size tester, test sieve machine DZS200, Kost Cup, 4339BHighResistanceMeter leakage current tester, E4980APrecisionLCRMeter capacity tester.
1.1.2 Experimental raw materials
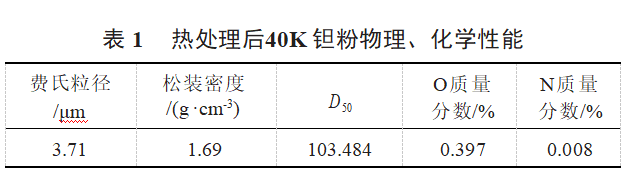
Raw material tantalum powder is vacuum heat-treated 40K tantalum powder, and the relevant physical and chemical properties are shown in Table 1.
Raw material magnesium chips are used commercially available magnesium chips with a particle size of 1700 μm, a purity (mass fraction) of not less than 99%, a Fe mass fraction of not more than 0.003%, and a Si mass fraction of not more than 0.003%.
1.2 Magnesium doping amount test
Weigh 5kg raw tantalum powder respectively, according to the oxygen mass fraction of raw materials, theoretically 1g of oxygen needs 1.5g of magnesium chips, doped with the theoretical amount of 0.5,1.0, 1.5, 2.0, 3.0, 4.0 times the magnesium chips, oxygen reduction temperature of 840 ° C, holding time of 2h, pumping magnesium time of 3h, to be passivated by the oxygen reduction, out of the furnace, according to the same pickling process to deal with the samples of A1, A2, A3, A4, A5, A6, and its physical, chemical and electrical properties of the analysis. The upper, middle and lower tantalum powder after the oxygen reduction of A1, A3 and A6 were sampled, and the samples were numbered as A11, A12, A13, A31, A32, A33, A61, A62 and A63, and analyzed for chemical properties.
1.3 Oxygen reduction temperature test
raw tantalum powder were weighed 5kg, mixed with the theoretical amount of 1.5 times the amount of magnesium chips, oxygen reduction temperature of 800, 840, 880, 920 ℃, respectively, the holding time of 2h, pumping magnesium time for 3h, to be passivated by the oxygen reduction, out of the furnace, according to the same pickling process, to get samples of B1, A3, B3, B4, for physical, chemical, and electrical properties of the analysis. Analyze the physical, chemical and electrical properties. The upper, middle and lower tantalum powder after oxygen reduction and discharging of B1 and B4 were sampled and numbered as B11, B12, B13, B41, B42 and B43, respectively, and analyzed for their chemical properties.
1.4 Pumping time test
raw material tantalum powder weighing 5kg, mixed with the theoretical amount of 1.5 times the magnesium chips, oxygen reduction temperature of 840 ℃, holding time of 0.5h, pumping time of magnesium for 4.5h, to be the oxygen reduction passivation, out of the furnace, according to the same pickling process above to get the sample C1, and its physical, chemical, and electrical properties of the analysis. Sample C1 tantalum powder after oxygen reduction out of the upper, middle and lower layers were sampled, numbered C11, C12, C13, respectively, and analyzed for their chemical properties.
2 Results and analysis
2.1 The effect of different magnesium doping amount, temperature and evacuation time on the physical properties of tantalum powder
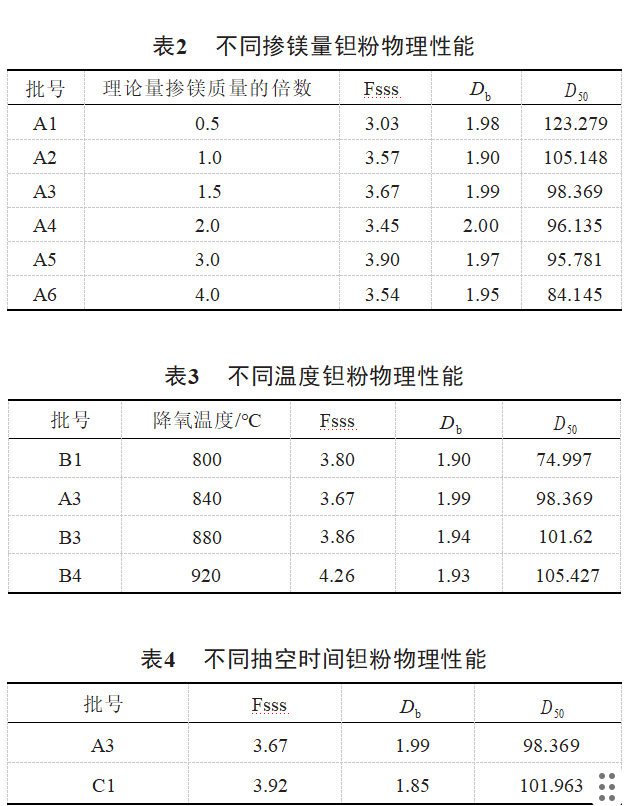
The comparison of the physical properties of samples A1, A2, A3, A4, A5, A6 is shown in Table 2, the comparison of the physical properties of samples B1, A3, B3, B4 is shown in Table 3, and the comparison of the physical properties of samples A3 and C1 is shown in Table 4. As can be seen in Table 2, the D50 of the product shows a tendency to decrease with the increase of the amount of magnesium doping. Fischer's particle size (Fsss), bulk density (Db) did not show significant changes. As the magnesium reduction process is exothermic, with the increase of magnesium doping, the reaction between magnesium and Ta2O5 in the furnace is more intense, resulting in the agglomeration and diffusion of fine particles without oxide film on the surface of the particles, and the D50 of tantalum powder decreases. As can be seen from Table 3, with the increase in temperature, the D50 of the product shows an increasing trend, Fischer's particle size, bulk density before 880 ℃ did not show significant changes, Fischer's particle size at 920 ℃ showed significant growth, magnesium at 900 ℃, its vapor pressure of 13332Pa, above 900 ℃, magnesium fugitive serious, higher temperatures can make the primary particles of tantalum powder agglomerates grow into secondary particles [10]. As can be seen from Table 4, the process of sample C1 reduces the liquid-solid sintering process, reduces the neck of the grain boundary to the direction of the small particles swept, the small particles dissolved in the liquid phase after the precipitation on the surface of the large particles and the grain boundary of the liquid film migration, reduces the liquid magnesium-tantalum powder particles agglomerated to grow up inhomogeneous phenomena, and the product of the Fischer's particle size is slightly larger, and the loose loading density is slightly smaller.
2.2 The effect of different magnesium doping, temperature, pumping time on the chemical properties of tantalum powder
2.2.1 The effect of different magnesium doping, temperature, pumping time on the chemical properties of tantalum powder after oxygen reduction
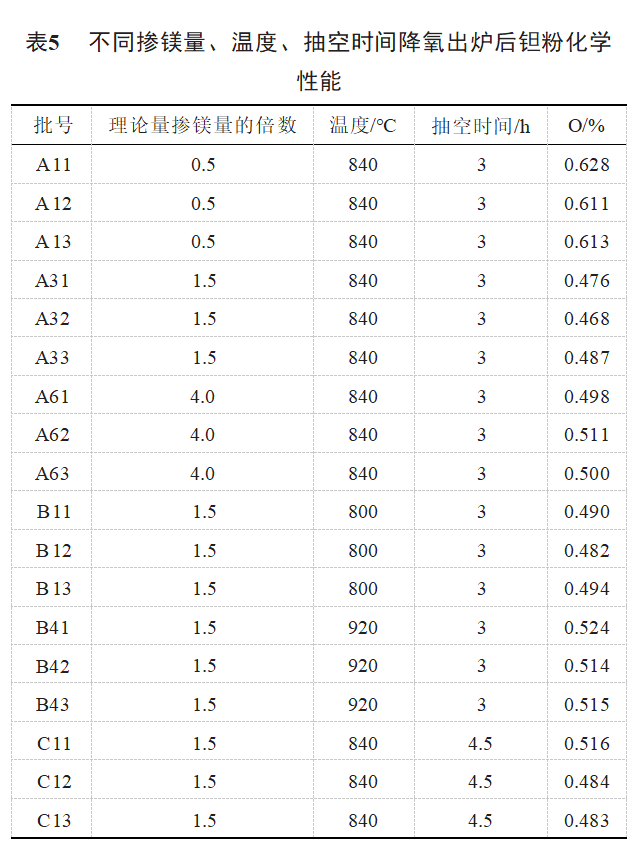
Samples A11, A12, A13, A31, A32, A33, A61, A62, A63, B11, B12, B13 physical properties comparison is shown in Table 5, Table 5 shows that, after oxygen reduction under different process conditions, the oxygen mass fraction after oxygen reduction has a marked Rise, magnesium deoxygenation reaction process produces MgO and excess magnesium metal in the tantalum powder passivation to generate MgO, tantalum powder after high temperature deoxygenation without oxide film fresh surface in the passivation process will re-absorb oxygen, ultimately leading to the lowering of the oxygen out of the oxygen increase is more.
2.2.2 Effect of different magnesium doping amount, temperature and evacuation time on the chemical properties of tantalum powder
The comparison of the chemical properties of samples A11, A12, A13, A31, A32, A33, A61, A62, A63, B11, B12, B13 is shown in Table 6, the comparison of the chemical properties of samples B1, A3, B3, B4 is shown in Table 7, and the comparison of the chemical properties of samples A3, C1 is shown in Table 8.
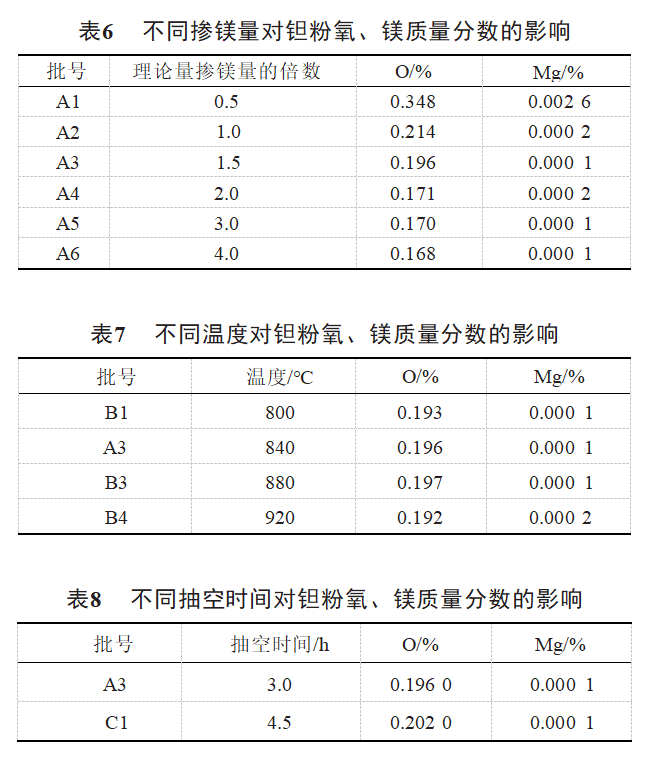
As can be seen from Table 6, when the magnesium addition is less than the theoretical amount, the oxygen and magnesium mass fractions are obviously high. When the amount of magnesium added is insufficient, a large amount of Ta2O5 is not reduced with the generated MgO to generate Mg4Ta2O9, tantalum metal wrapped to form a sintered body [11], which ultimately leads to high oxygen and magnesium content of tantalum powder. With the increase of the magnesium doping amount of magnesium vapor concentration of the reaction system is larger, can be fully reacted with the Ta2O5 layer on the surface of the tantalum powder, the reaction is more intense, more thorough, the oxygen mass fraction has a tendency to decline. When the amount of magnesium doping to 2 times, the oxygen content of tantalum powder is basically unchanged, the reason is to be able to maintain the consumption of magnesium thermal reduction reaction [12], the oxygen reduction space is not much. As can be seen from Table 7, the oxygen mass fraction is basically unchanged with the increase of temperature. The magnesium doping and temperature as an important index affecting the effect of oxygen reduction, in the case of sufficient magnesium content, the oxygen mass fraction does not change much in a certain temperature range. From Table 8, it can be seen that in the range of 3-5h of pumping time, the extension of pumping time has little effect on the oxygen and magnesium mass fraction. The evacuation is to pump the excess unreacted magnesium vapor from the pores of tantalum powder particles to the outside of tantalum powder in the early stage, and to achieve the separation of excess magnesium and tantalum powder through the condensation in the cooling zone in the later stage, due to the fact that the evacuation time in the early stage is sufficient to make the excess magnesium vapor discharge to the outside of oxygen-reducing tantalum powder, and the simultaneous holding time can make the magnesium react fully with the Ta2O5 layer, and the final evacuation time is in the range of 3~5 h. The prolongation of evacuation time does not have much effect on oxygen and magnesium mass fraction. , magnesium mass fraction has little effect.
2.3 The effect of different magnesium doping, temperature, pumping time on the electrical properties of tantalum powder
The electrical properties were tested according to the FTA400 conditions in the GB/T3137-2020 tantalum powder electrical properties test method.
2.3.1 The effect of different magnesium doping, temperature and pumping time on the electrical properties of tantalum powder
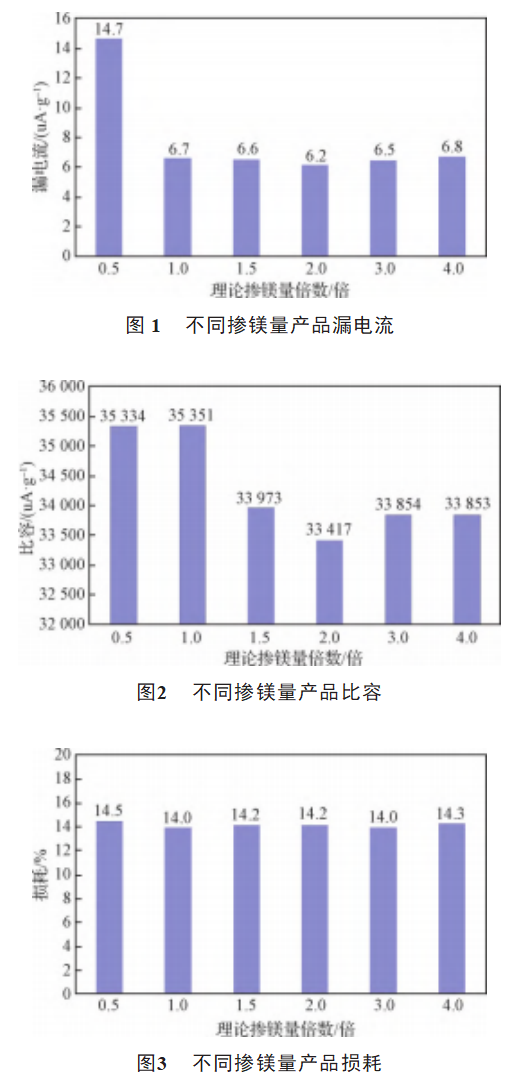
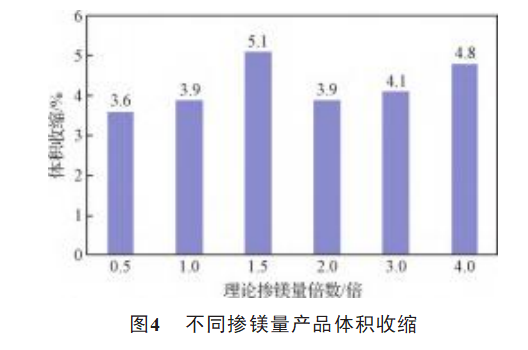
The comparison of the electrical properties of samples A1, A2, A3, A4, A5, A6 is shown in Fig. 1, Fig. 2, Fig. 3, and Fig. 4. It can be seen in Fig. 1, when the magnesium doping amount is insufficient the leakage current of the product is large, and the oxygen mass fraction of the product is high, and the residual oxygen diffuses into the lead wire to make the tantalum wire lead brittle, resulting in the leakage current increasing sharply. Excessive oxygen is easy to make the tantalum powder surface crystal oxide film, constantly squeezing the surrounding amorphous oxide film, destroying the amorphous oxide film, resulting in uneven thickness, the crystal oxide film and amorphous oxide film between the existence of small cracks, resulting in the capacitor leakage current rises [13]. As can be seen from Figure 2, the magnesium doping amount increased to 1.5 times when the specific capacitance of the product has a substantial decrease in the specific capacitance of the product within a certain range is proportional to the oxygen mass fraction, combined with Table 4 shows that when the magnesium doping amount of 1.5 times, the oxygen mass fraction changes significantly, so that the product specific capacitance has a significant change. From Fig. 3 and Fig. 4, it can be seen that with the increase of magnesium doping, the loss and volume shrinkage of the product did not change significantly.
2.3.2 The effect of different temperatures on the electrical properties of tantalum powder
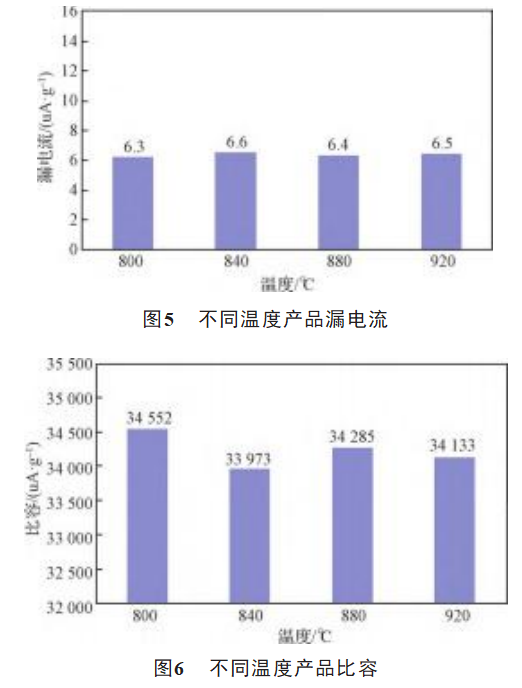
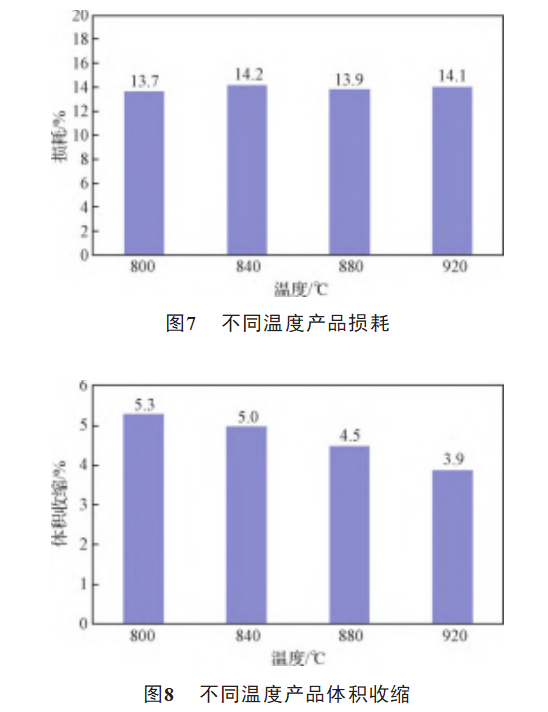
The comparison of the electrical properties of samples B1, A3, B3 and B4 are shown in Figure 5, Figure 6, Figure 7 and Figure 8. For the sintering process, the increase in sintering temperature will definitely reduce the impurity content in the matrix, improve the quality of the dielectric oxide film and reduce the loss. However, it will also lead to a decrease in the porosity of the anode matrix, narrowing the pore diameter, thus increasing the equivalent series resistance of the electrolyte and increasing the loss. For high specific capacity tantalum powder, attention should be paid to the balance between the two in the sintering process. As shown in Figure 5, Figure 6 and Figure 7, the leakage current, specific capacitance and loss of the product did not change significantly with the increase in the temperature of oxygen reduction. As can be seen from Figure 8, with the increase in temperature the volume shrinkage of the product is a decreasing trend [14], sintering is to make the original independent of each other tantalum powder particles in high temperature, vacuum conditions to achieve partial mutual fusion, the higher the temperature, the more obvious the sintering effect of the powder [15], the increase in the strength of the particles, the reduction of the volume contraction, at a temperature of more than 880 ° C, the reduction of volume contraction is obvious. Therefore the temperature of the production process should be controlled below 880°C.
2.3.3 Effect of different pumping time on the electrical properties of tantalum powder
Comparison of the electrical properties of samples A3 and C1 is shown in Figure 9, Figure 10, Figure 11 and Figure 12.
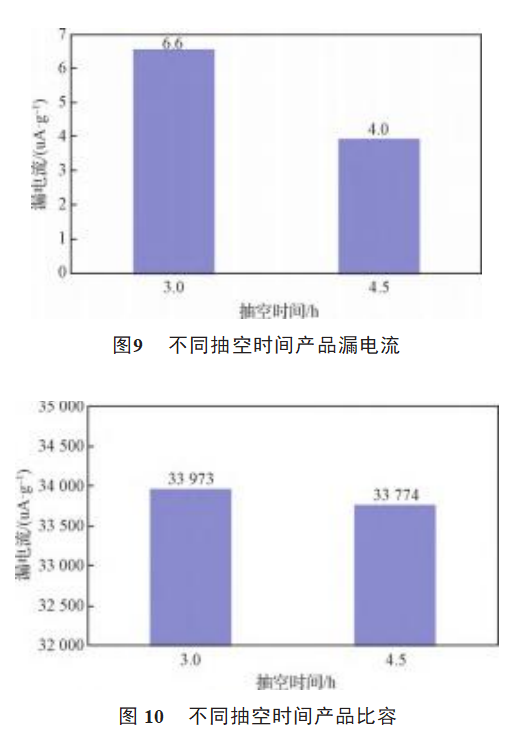
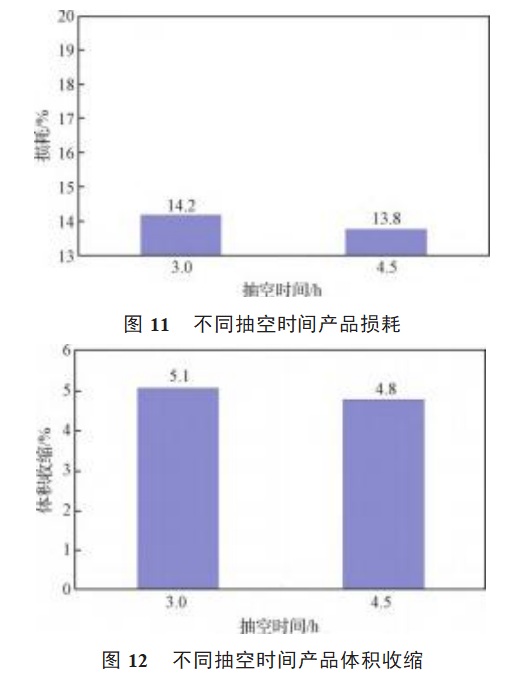
As can be seen from Figures 9 and 11, the leakage current and loss of sample C1 is obviously small, and the pumping time is 3-5h. Extending the pumping time reduces the liquid-solid sintering time and increases the gas-solid reaction time, magnesium vapor and tantalum oxide in tantalum powder fully contact and react [15], the reaction process is more uniform. The thickness of the particle oxide film is uniform, and the leakage current and loss of the product are small. From Figures 10 and 12, it can be seen that prolonging the pumping time has little effect on the specific volume and volume shrinkage of the product.
3 Conclusion
(1) Insufficient magnesium content will lead to high oxygen and magnesium products, leakage current is large, the production process of magnesium additions need to be higher than the theoretical amount, at least 1 times the theoretical amount.
(2) With the increase of temperature, the volume shrinkage has a tendency to decrease, in the temperature above 880 ℃, the volume shrinkage decreases significantly, the temperature of the production process should be controlled below 880 ℃.
(3) Pumping time at 3~5h, reducing the liquid-solid sintering time and increasing the gas-solid reaction time to 4.5h can make the leakage current and loss of the product obviously low.
Reference: Powder Metallurgy Industry Volume 35, Issue 3 June 2025 DOI: 10.13228/j.boyuan.issn1006-6543.20240040
Stardust's spherical tantalum powders are prepared by RF plasma with high purity (low oxygen and nitrogen impurities), >95% ultra-high sphericity, and uniform particle size distribution with excellent flowability. It is suitable for 3D printing and powder metallurgy. Its high specific surface area and controllable performance enhance the printing efficiency and densification of products, which are widely used in aerospace, medical devices (e.g. orthopedic implants) and electronic components, and comply with YY/T0966-2014 and other medical standards, as well as environmental protection advantages. For more information, please contact our professional staff, Manager Zheng +86 13318326187.


