Determination of silicon in tantalum alloys by microwave digestion - inductively coupled plasma emission spectrometry
Release time:
2025-07-01
Among the refractory metal materials, tantalum metal (Ta) and tantalum alloys have high melting points, very low tough-brittle transition temperatures, good plastic processing and molding ability, excellent corrosion resistance, wear resistance, creep resistance and high-temperature mechanical properties [1~3], and they are widely used in many high-tech fields, such as aerospace, nuclear, metallurgy, chemical and defense [4, 5]. and national defense and many other high-tech fields [4, 5]. These new technological fields have very strict requirements on the physicochemical properties of tantalum and tantalum alloys, and the content of various impurities in the alloys affects the physicochemical properties of tantalum and tantalum alloys to a large extent. Therefore, it is very important to find an accurate and reliable analytical test method to analyze the impurity elements in tantalum and tantalum alloys. Silicon is an important impurity in tantalum alloys, and its content directly affects the performance of tantalum alloys. Therefore, accurate analysis of silicon in tantalum alloys is crucial. Traditional silicon analysis methods mainly include chemical analysis and instrumental analysis. Chemical analysis methods mainly include fluorosilicic acid titration and molybdenum blue spectrophotometry and gravimetric method, while instrumental analysis methods mainly include inductively coupled plasma atomic emission spectrometry (ICP-AES) and inductively coupled plasma mass spectrometry (ICP-MS). Due to the low silicon content in tantalum alloys, ICP-AES has become the mainstream method for silicon analysis in tantalum alloys due to its advantages of high sensitivity, high precision, wide linear range and strong anti-interference ability.
Due to the characteristics of tantalum alloy material itself, it is necessary to use hydrofluoric acid to dissolve the sample, but hydrofluoric acid dissolution of the sample for the elemental silicon has strict temperature requirements, the temperature needs to be kept below 60 ℃, otherwise it will lead to the loss of silicon, and the temperature is too low, the tantalum alloy dissolution time is too long, and it can't satisfy the actual analytical needs. Microwave digestion method as a simple and efficient method of dissolving samples, just to meet the requirements of this analysis. The microwave digestion method accelerates the dissolution of samples by using the heat generated by microwaves in a closed digestion tank to carry out strong thermal convection, and only acid is added as a solvent in the experiments without introducing other impurity elements, with complete dissolution of the samples and low blank value [12, 13]. Compared with the traditional pretreatment methods, microwave digestion has the advantages of saving time and labor, low acid consumption, low pollution and no volatile loss [14]. In the present work, tantalum alloy was dissolved in nitric acid and hydrofluoric acid by microwave digestion in a closed chamber, which greatly improved the dissolution efficiency of tantalum alloy, and the determination of silicon by inductively coupled plasma atomic emission spectrometry (ICP-ATES), which realized the rapid and accurate analysis of silicon in tantalum alloy. In this paper, the precise analysis method of silicon in tantalum alloys is discussed, which provides a scientific basis for the production and application of tantalum alloys. In the future research, the experimental method will continue to be optimized to improve the analytical efficiency and accuracy, so as to meet the higher requirements for tantalum alloy performance in high-tech fields. Meanwhile, research on the analytical methods of other impurity elements will also be paid attention to provide more technical support for the wide application of tantalum metal and tantalum alloys.
1 Experimental part
1.1 Main instruments and working conditions
ICAP6300 Inductively Coupled Plasma Emission Spectrometer (Thermo Fisher Co., Ltd., USA), equipped with hydrofluoric acid-resistant sampling system, with a resolution of <0.006 mm (at 200 nm). Working conditions: sample cleaning time of 30s, analytical pump speed of 50r/min, power of 1150W, carrier gas flow rate of 0.75L/min, auxiliary gas flow rate of 0.5L/min.
EIKO ultrapure water machine: ultrapure water, 10~16MΩ,25℃ (Chengdu Corning Technology Development Co., Ltd.).
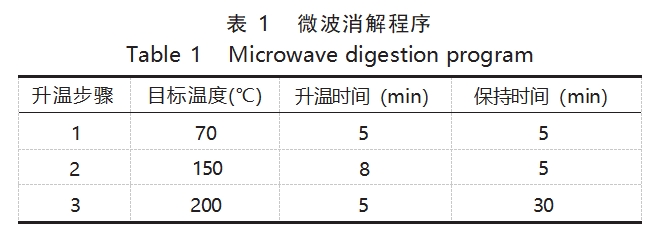
An Eiyao M6 microwave digestion instrument was used to dissipate the metal tantalum under airtight conditions with step-by-step temperature increase, and the microwave digestion procedure is shown in Table 1.
1.2 Main reagents
Nitric acid (ρ=1.42g/mL); Hydrofluoric acid (ρ=1.14g/mL). Argon (volume fraction not less than 99.99%).
Silicon standard solution: GSBG62007-90 (1401) 1000 μg/mL, 10% HCl medium, provided by the National Iron and Steel Materials Testing Center Iron and Steel Research Institute. All experimental water was ultrapure water (10~16MΩ).
1.3 Experimental method
Weigh 0.5000g of samples dried at 105 ℃ in the microwave digestion inner tank, respectively, add 3mL nitric acid, 4mL hydrofluoric acid and 4mL water; cover the lid of the inner tank, fasten the explosion-proof membrane, place it into the outer tank, tighten it and then put it into the microwave digestion instrument, according to the heating procedure in Table 1 for microwave digestion. Remove after completion, soak in cold water for more than 2h, open in the fume hood; transfer the solution into a 100mL plastic volumetric flask and clean the ablation tank with water, set the volume and shake well for use. Do two experiments in parallel and take the average of the results; do the blank experiment with the sample.
1.4 Preparation of standard solution series
Weigh four copies of metal tantalum equivalent to the sample, were placed in the microwave digestion tank, and were taken 0.00mL, 1.00mL, 5.00mL, 10.00mL concentration of 100.0μg/mL of silicon standard solution in the microwave digestion tank, according to the steps of 1.3 processing, cooling and then transferred to a fixed volume, to obtain a series of standard solutions. The amount of silicon contained in this standard solution was 0 μg/mL, 1.00 μg/mL, 5.00 μg/mL and 10.00 μg/mL, in that order.
2 RESULTS AND DISCUSSION
2.1 Optimization of microwave dissolution procedure
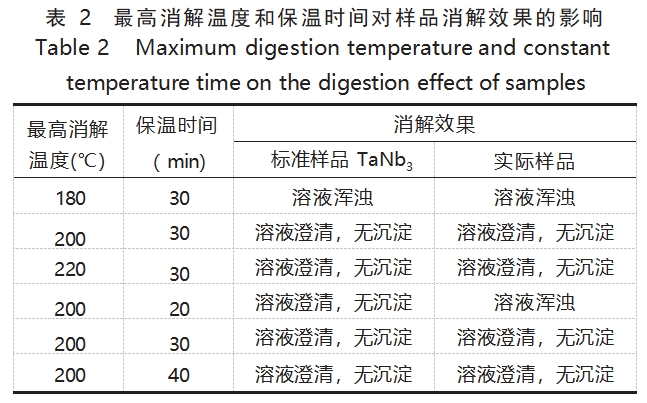
The dissolution efficiency of the samples was closely related to the maximum temperature as well as the holding time. Generally speaking, in the microwave digestion program, the higher the maximum digestion temperature and the longer the holding time, the better the digestion effect. This work aims to accelerate the dissolution of tantalum alloys with the expectation of achieving the best in terms of time and money costs, so the experiments were carried out to optimize the maximum temperature as well as the holding time in the microwave dissolution procedure. The experiments were carried out with the standard TaNb3 and the actual samples respectively, and 3mL of nitric acid and 4mL of hydrofluoric acid were added for microwave dissolution. Firstly, fix the holding time, change the maximum temperature, the experimental phenomenon can be seen, the dissolution temperature is not high enough will inevitably affect the dissolution of tantalum alloy; similarly, fix the maximum temperature, change the holding time of the experiments, the experimental phenomenon also shows that the dissolution of tantalum alloy has a certain requirement on the holding time. Comprehensively, the experiment selected the highest temperature of dissolution for 200 ℃, holding time for 30min.
2.2 Optimization of hydrofluoric acid dosage
Hydrofluoric acid is one of the common components of the dissolution solution, which can effectively destroy the structure of tantalum, so that the elements embedded in the precipitation, and therefore in the tantalum alloys in the microwave dissolution of hydrofluoric acid must be used. The dosage of hydrofluoric acid has a crucial effect on the dissolution efficiency of tantalum alloy, so the experiment optimized the dosage of hydrofluoric acid. The amount of nitric acid was fixed at 3 mL, and the amount of hydrofluoric acid was changed at the same time. The results of Si determination in TaNb3 samples under different conditions are shown in Table 3.
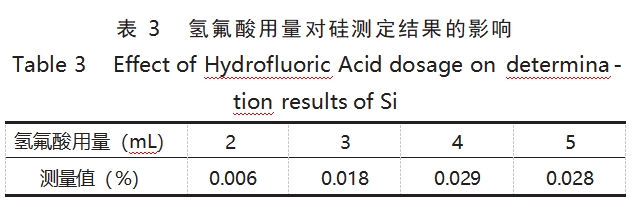
The experimental phenomena and the data in Table 3 show that under the conditions of high temperature and high pressure, the insufficient amount of hydrofluoric acid will lead to the samples can not be completely dissolved, i.e., in the case of adding 2 mL of hydrofluoric acid, the determination value of Si is obviously low; elevating the amount of hydrofluoric acid can promote the dissolution of the samples, and the determination result of Si tends to be stabilized when the dosage is 4~5 mL. Therefore, the experiment chooses to add 4mL of hydrofluoric acid for microwave digestion.
2.3 Optimization of nitric acid dosage
Nitric acid has strong oxidizing property and is one of the common components of the digestion solution, which can effectively dissolve most of the alloy samples, so the experiment explored the influence of the dosage of nitric acid on the efficiency of tantalum alloy digestion, and the same test was carried out on the standard substance TaNb3, and the fixed hydrofluoric acid dosage of 4mL, and the results of the experiment are shown in Table 4.
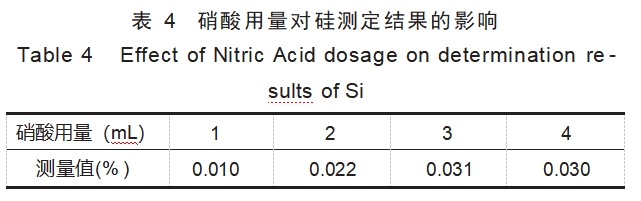
The experimental phenomena and the data in Table 4 show that the insufficient dosage of nitric acid will also lead to the samples can not be completely dissolved, that is, in the case of adding 1mL of nitric acid, the determination value of Si is obviously low; increase the dosage of nitric acid can promote the dissolution of the samples, and when the dosage of 3~4mL, the results of the determination of Si tends to be stabilized. Therefore, the experiment determined that the dosage of nitric acid in the digestion solution was 3mL.
2.4 Selection of analytical spectral lines and calibration curves
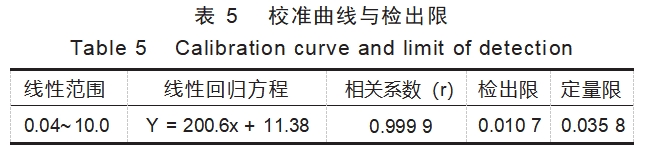
Considering that the presence of a large number of tantalum elements may cause interference in the detection of silicon, therefore, the optimal analytical spectral lines were selected in accordance with the criteria of little or no interference with the elements to be measured, low background and high sensitivity. Two analytical lines (251.612 nm, 21.412 nm) for silicon were selected for comparison, and finally 251.612 nm with higher sensitivity and better calibration curve coefficients was chosen as the analytical line for this experiment. The intensity of the emission spectrum of silicon in the series of standard solutions was detected under the selected instrumental conditions, and the calibration curve was plotted with the mass concentration of silicon (mg/L) as the horizontal coordinate and the corresponding intensity of the emission spectrum as the vertical coordinate. The linear ranges, regression equations and correlation coefficients are shown in Table 5. The blank was determined continuously for 11 times, and the detection limit of silicon was calculated by three times of the standard deviation of the determination results, and the quantification limit of silicon was calculated by 10 times of the standard deviation of the blank, which is shown in Table 5.
2.5 Accuracy and Precision
In order to validate the accuracy and precision of the present method, the tantalum alloy standard material (TaNb3) was selected, and analyzed according to the experimental method, and the results are shown in Table 6: Three experiments were carried out respectively, and the silicon The measured values coincided with the identified values, and the relative standard deviation (RelativeStan-dardDeviation, RSD, n=3) of the measurement results was between 2.3% and 3.5%, indicating that the method has high accuracy and good precision.

2.6 Analysis of actual samples
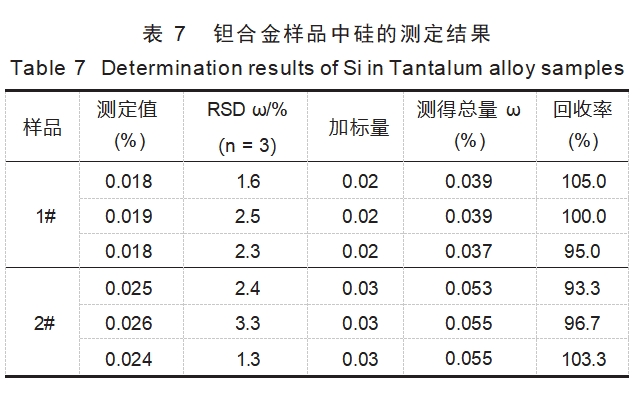
Two tantalum alloy samples were selected and analyzed according to the experimental method, and the results are shown in Table 7: The relative standard deviations of the determination results were between 1.3% and 3.3%, and the spiked recoveries were between 93.3% and 105%, which indicate that the method can accurately determine the silicon in tantalum alloy samples.
3 Conclusion
In this work, an analytical method for the determination of silicon in tantalum alloy samples by microwave digestion-inductively coupled plasma emission spectrometry was proposed. Compared with the traditional water bath method, this method greatly enhances the dissolution speed of the sample and improves the analytical efficiency of the sample. The dosage of hydrofluoric acid and the selection of analytical spectral lines were optimized; the standard samples as well as the actual samples were examined under the optimized experimental conditions, and the results showed that the method has good accuracy and precision, and the analysis is rapid, which can be used for the accurate determination of silicon in tantalum alloy samples.
Reference: Zhang Hao et al: Determination of Silicon in Tantalum Alloys by Microwave Ablation-Inductively Coupled Plasma Emission Spectrometry
Stardust Technology (Guangdong) Co., Ltd. focuses on the research, development, and production of high-end spherical metal powders, with its core products covering tantalum, tungsten, molybdenum, niobium, and other rare and refractory metals and their alloy powders, and tungsten-molybdenum-tantalum-niobium refractory and high-entropy alloy powders, which are all prepared by RF plasma spheronization technology, and are equipped with high purity, The powders are prepared by radio frequency plasma spheronization technology, and are characterized by high purity, good sphericity, no satellite balls, excellent fluidity and uniform particle size distribution. The spherical tantalum powder has passed the international standard certification in the field of medical implantation, which is the ideal material for orthopedic 3D printing implants; refractory high entropy alloy powder serves in the high-end fields of national defense, aerospace and so on. The company's technical team originated from Guangdong Academy of Sciences, with more than 30 years of scientific research accumulation, led the formulation of a number of national and industry standards, and realized the localization of the mass production of medical spherical tantalum powder. Welcome to contact our professional and technical staff, Manager Zheng +86 13318326187.


