Research Progress on Surface Modification and Functionalization of 3D-Printed Porous Tantalum
Release time:
2025-09-04
The aging population has led to a year-on-year increase in the prevalence of musculoskeletal diseases. Among these, bone defects represent the most common orthopedic condition, characterized by partial skeletal loss caused by severe limb trauma, bone infections, tumors, and osteomyelitis. Globally, over 2 million bone graft surgeries are performed annually [1]. Large-segment bone defects or critical-sized defects remain challenging clinical issues. When bone defects exceed the self-healing capacity of the skeleton, intervention with bone repair materials implanted into the defect site is required [2-3]. Current bone defect repair materials primarily include autogenous bone, allograft bone, and synthetic bone substitutes. Autogenous bone faces limitations in donor availability and risks donor site injury, while allograft bone may induce complications such as immune rejection and cross-infection of diseases [4]. Among numerous synthetic bone repair materials, tantalum (Ta) metal holds broad application prospects in post-traumatic bone defect repair due to its excellent biocompatibility, suitable mechanical properties, superior osteoconductivity, and osteoinductivity [5].
Compared to traditional medical titanium alloys, Ta implants demonstrate superior corrosion resistance, biocompatibility, and osteogenic properties [6-7]. However, with deeper clinical application, Ta implants have gradually revealed a series of drawbacks. For instance, Ta's high density (16.68 g/cm³) and high melting point (3200 K) result in poor processability. Additionally, limitations such as the stress shielding effect, surface biocompatibility issues, and high cost restrict its widespread adoption.
Porous Ta fabricated via additive manufacturing (AM) exhibits a porous structure and elastic modulus similar to human cancellous bone. On one hand, its open interconnected porous structure facilitates bone tissue ingrowth, enabling effective early biological fixation. On the other hand, its elastic modulus close to that of human bone tissue reduces the stress shielding effect, making it suitable as a bone replacement material for weight-bearing sites [8-9]. Compared to Zimmer's TrabecularMetal™ trabecular metal implants produced via chemical vapor deposition (CVD), AM porous Ta implants offer advantages such as simplified fabrication, controllable structure, and customizable design [10]. Precise and personalized control over surface chemistry, topological structure, and mechanical properties of such customized implants will significantly promote bone tissue regeneration, meeting the diverse needs of patients with bone defects [9,11]. Nevertheless, research on AM porous Ta implants remains in its infancy both domestically and internationally. As the volume of orthopedic implant surgeries increases, patients continue to face risks such as poor osseointegration, bacterial infection, and surface inertness of implants. In recent years, researchers have explored surface modification techniques and topological structure optimization to functionalize AM porous Ta implants. This paper reviews various surface modification methods for AM porous Ta and their characteristics, summarizes the impact of topological structure design on biofunctionalization, and concludes with prospects for the development and clinical application of functionalized AM porous tantalum.
1 Surface Modification Methods for Porous Tantalum
The surface properties of biomaterials directly influence biocompatibility and tissue integration capacity. Surface morphology, roughness, chemical composition, and charge density affect material–cell interactions, thereby improving the bonding quality at the bone–implant interface and promoting initial implant stability [12–13]. Current surface modification techniques for porous Ta primarily include alkaline heat treatment, anodic oxidation, biomimetic inorganic coatings, organic coatings, sustained-release drug-eluting coatings, and antimicrobial coatings. Table 1 summarizes various surface modification methods for AM porous tantalum along with their advantages and disadvantages [14-21].
1.1 Anodic Oxidation
Anodic oxidation offers low cost, simple processing, and easy manipulation and control of nanoscale morphology, making it commonly employed for preparing nanoporous structures in various metal oxide systems. Specifically, using tantalum (Ta) as the anode and platinum (Pt) as the cathode, redox reactions occur in the electrolyte solution. During this process, nanotubes, nanopores, and nanocavities can form on the Ta surface, primarily composed of Ta₂O₅ [16-17]. . Nanoscale porous oxide films increase the contact area between the material and bone tissue, thereby enhancing the wettability and adsorption of proteins, ions, and cells. Most studies have focused on the physical, chemical, and biological properties of Ta and porous Ta surfaces after anodization. Wang et al. [18] prepared highly ordered nanotube films on pure Ta surfaces via anodization. They found that Ta₂O₅ nanotube films exhibit excellent corrosion resistance and enhance protein adsorption on the Ta surface. This promotes the adhesion, proliferation, and differentiation of rabbit bone marrow mesenchymal stem cells, while elevating the expression levels of osteogenesis-related factors (alkaline phosphatase (ALP), type I collagen (Collagen–I), and osteocalcin).
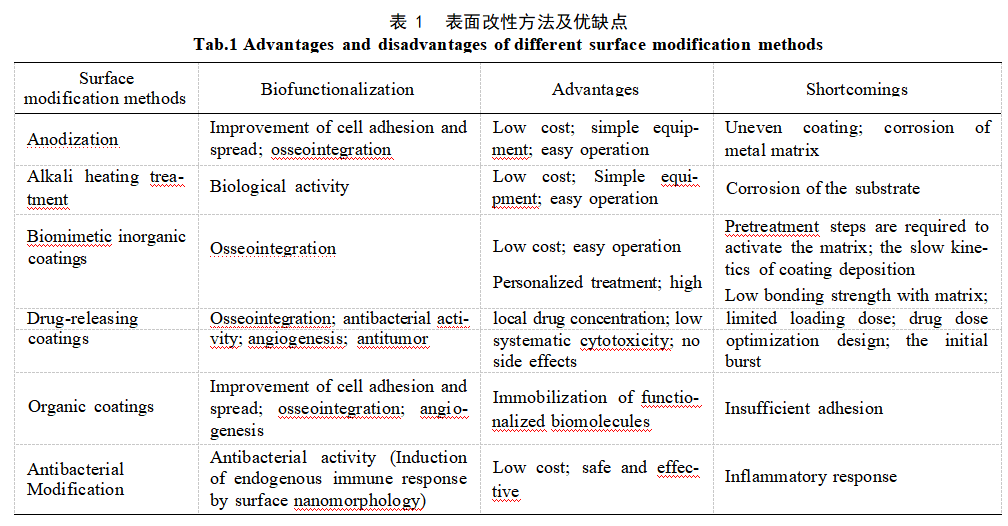
Bone exhibits a hierarchical structure spanning macro- to nanoscale levels (Figure 1a), primarily composed of softer collagen and harder apatite minerals. This dual composition confers both strength and toughness, making bone a lightweight material capable of bearing substantial loads [22]. Ideal orthopedic implants should mimic the microstructure and mechanical environment of human bone tissue. Mimicking natural bone has become a key strategy for developing novel bone tissue engineering scaffold materials. To this end, most studies modify the surface morphology of porous Ta by controlling anodic oxidation process parameters such as voltage, electrolyte composition, temperature, and current to obtain micro/nano-porous layers similar to human bone. Zhang et al. [23] employed selective laser melting (SLM) to fabricate micro-gradient porous Ta scaffolds and generated Ta nanotubes on the porous Ta surface via anodization (Figure 1b). Experimental results demonstrated that the micro/nano-layered structure of porous Ta promotes osteogenesis both in vitro and in vivo. Filopodia of mouse embryonic pre-osteoblasts (MC3T3–E1) could enter the Ta nanotubes and form interlocking unit structures. Compared to the untreated porous Ta group, osteogenic marker genes were upregulated by approximately 3–4-fold. Seven weeks after implantation into rabbit femurs, early osseointegration performance was significantly enhanced compared to the control group. Additionally, another study [24] demonstrated that Ta₂O₅ nanotubes prepared on microporous Ta surfaces promote adhesion and proliferation of human bone marrow-derived mesenchymal stem cells more effectively than bare microporous Ta, with osteogenic genes upregulated by 1.5–2.1-fold. Ta₂O₅ nanotubes can also form in acid-free electrolytes. Fialho et al. [25] prepared a regular, ordered porous Ta₂O₅ surface via a one-step anodization process in a HF-free electrolyte (ethylene glycol, water, ammonium fluoride). However, whether its physical and biological properties offer advantages over oxides prepared using HF-containing electrolytes requires further investigation. From a biomechanical perspective, nanoporous surfaces effectively regulate cell growth and orientation. From a physical biomimetic standpoint, the surface structure of these Ta₂O₅ nanotube arrays mimics the complex geometry of natural bone tissue, providing a porous network structure conducive to cell growth. In future clinical applications, 3D-printed porous Ta with biomimetic micro/nano-gradient structures holds great promise for enhancing implant osseointegration.
Although studies on constructing nanotubes on bulk Ta surfaces via anodization have been extensively reported, research on anodizing porous Ta surfaces remains scarce. On one hand, the growth mechanism of nanotube films on Ta metal surfaces is still being explored; on the other hand, the complex three-dimensional structure within 3D-printed porous metals leads to uneven distribution of the anodic electric field. The formation of nanotube films during oxidation arises from the competitive interplay between the continuous formation and dissolution of TaOx. Nanotubes undergo a dynamic process of continuous growth and dissolution during anodization. This leads to non-uniform phenomena on porous metal surfaces where locally formed nanotubes coexist with collapsed ones in other regions under identical oxidation durations. Therefore, further optimization of anodic oxidation parameters for porous metals is required to construct uniform nanotube films.
1.2 Alkali Heat Treatment
Alkaline heat treatment is a simple and economical surface modification technique commonly used to enhance the surface bioactivity of metallic bone repair materials. This process involves immersing the metal material in a NaOH solution followed by high-temperature heat treatment. During this procedure, the tantalum oxide layer on the surface of the Ta implant reacts sequentially with the NaOH solution to form an amorphous layer of sodium tantalate, which is then stabilized through heat treatment [27-28]. Only at appropriate NaOH concentrations can tantalum's biocompatibility be ensured while simultaneously enhancing its bioactivity. Lin et al. [29] observed the formation of crystalline sodium tantalate (Na₅Ta₁₁O₃) on the surface of tantalum after alkaline heat treatment in 1.0 M NaOH, which severely impaired the proliferation activity of MG63 cells. Alkaline heat treatment is typically employed as a pretreatment for other surface modification techniques to enhance material surface bioactivity. For instance, the negatively charged surface of alkali-heat-treated Ta can bind with positively charged Ca²⁺ in physiological fluids to form amorphous calcium phosphate, thereby improving early osseointegration capacity of implants.
1.3 Bioinspired Inorganic Coatings
Bioinspired inorganic coatings primarily refer to calcium phosphate (CaP)-based coatings, widely used to modify metal implant surfaces due to their inorganic composition similar to natural bone. CaP coatings on Ta surfaces are typically obtained through two methods: using organic coatings (e.g., organosilanes, organophosphates) as apatite nucleation agents [30-31]; or via urea-mediated heterogeneous mineralization, where acidic hydroxyapatite solutions promote apatite nucleation and growth [32]. Activated Ti, Zr, Nb, or Ta metal surfaces can form biomimetic apatite in simulated body fluid (SBF) environments, whose crystal structure more closely resembles natural bone mineral than hydroxyapatite, tricalcium phosphate, or tetracalcium phosphate [33]. Barrère et al. [14] immersed porous Ta in 5× concentrated SBF at 37°C for 24 hours, forming a uniform amorphous calcium phosphate layer that served as a seeding layer for subsequent crystalline growth. Continued immersion in modified SBF containing crystal inhibitors (Mg²⁺, HCO₃⁻) yielded a 30μm-thick hydroxyapatite coating. Compared to unmodified porous Ta, porous Ta with the bio-mimetic calcium phosphate coating exhibited heterotopic ossification after 12 weeks of implantation in goat back muscles. When implanted into goat bone shafts, it significantly increased bone contact rate and markedly accelerated bone ingrowth velocity.
Combining alkali heat treatment with calcium phosphate deposition technology enables preparation of a more effective bio-mimetic calcium phosphate layer on the surface of Ta implants. Following alkaline heat treatment, the amorphous sodium tantalate formed on the Ta substrate surface exchanges with H₃O⁺ ions in SBF to generate Ta—OH groups. These groups react with Ca²⁺ and phosphate ions in SBF, promoting apatite nucleation on the Ta surface and forming amorphous calcium tantalate. Subsequently, the calcium tantalate further transforms into calcium phosphate [34]. The Ta—OH groups are critical for influencing the formation of apatite on the Ta surface, with their formation rate determining the rate of apatite layer formation. Miyazaki et al. [15] found that under identical conditions, alkaline heat treatment accelerates the formation of Ta—OH groups on the Ta surface.
1.4 Organic Coatings
Organic coating modification typically functionalizes biomaterials using cell adhesion molecules such as natural extracellular matrix proteins or short peptide sequences. These ligands interact with cells via integrin receptors, triggering cell adhesion and mechanotransduction signals while promoting cell migration, proliferation, and differentiation. The arginine–glycine–aspartic acid (RGD) motif, widely present in extracellular matrix proteins like fibronectin and osteopontin, specifically binds integrin subunits on osteoblasts and is extensively used for functionalizing metallic materials. Mas-Moruno et al. [35] immobilized RGD onto Ta surfaces via physical adsorption and alkylation, demonstrating superior cell adhesion compared to unmodified Ta. Wang et al. [36] applied a cyclic RGD-modified porous Ta scaffold to a rabbit radial phalanx staged bone defect model. Comparisons with uncoated porous Ta at 4, 8, and 16 weeks revealed significantly enhanced new bone formation and bone maturation.
Under weakly alkaline conditions upon exposure to air, dopamine (DA) can form polydopamine (PDA) nanocoatings on virtually any solid surface [37]. The presence of catechol groups in PDA structures enables rapid coverage of all inner surfaces within porous structures, making it a simple and highly efficient organic bio-coating material. PDA acts as a carrier for bioactive molecules by undergoing Michael addition or Schiff base reactions with biomolecules via its surface-rich active functional groups, thereby introducing bioactive metal ions. Sr and Mg, as essential trace elements for the human body, possess the ability to promote vascular and bone formation. Over 60% of Mg in the human body is concentrated in bones and teeth. Mg²⁺ can enter dorsal root ganglion (DRG) neurons, promoting calcitonin gene-related peptide (CGRP) release. This activates CGRP receptors on the surface of periosteal-derived stem cells (PDSCs), triggering CREB1 phosphorylation via cyclic AMP (cAMP) and enhancing the expression of osteogenic differentiation genes [38]. Ma et al. [19] successfully doped Mg²⁺ onto the surface of porous Ta scaffolds prepared from SLM via dopamine self-assembly, enhancing both in vivo and in vitro osteogenic and angiogenic activities. Sr²⁺ shares similar properties with Ca²⁺ and can thus participate in Ca²⁺-dominated osteogenic processes within bone metabolism. Moderate Sr²⁺ levels promote osteoblast proliferation and differentiation by activating calcium-sensitive receptors (CaSR), increasing OPG production, and reducing RANKL expression. This induces osteoclast apoptosis, stimulates bone formation, inhibits bone resorption, and increases bone mass [39-40]. Simultaneously, Sr²⁺ promotes early vascularization of biomaterials by modulating macrophage phenotypes. Cheng et al. [41] incorporated Sr²⁺ into porous Ta surfaces (PTD–Sr) via dopamine self-assembly. The Sr²⁺-doped porous Ta scaffold exhibited stable Sr²⁺ release over 14 days, enhancing expression of angiogenesis- and osteogenesis-related RNAs. In a rat condylar defect model, compared to the control group, the PTD–Sr scaffold demonstrated significantly enhanced new bone formation within the defect area and exhibited superior angiogenic behavior. However, critical issues such as the long-term stability and toxicity of PDA in vivo remain to be investigated.
1.5 Sustained-Release Drug Delivery Coatings
Sustained-release drug delivery coatings are typically constructed by modifying implant surfaces with growth factors, extracellular matrix proteins, peptides, or drugs to impart specific functionalities [42]. Porous Ta exhibits excellent corrosion resistance, making it an ideal drug-loading scaffold. Its microporous surface morphology not only enhances osseointegration through bone ingrowth but also enables functionalization via self-assembled monolayers and drug incorporation to achieve sustained release capabilities. Controlling the degradation of bioactive molecules through sustained-release drug delivery carriers enables continuous and effective drug release into adjacent bone tissue. This approach reduces side effects associated with conventional dosing and significantly enhances early stability between the implant and host bone. Drug binding to porous Ta is typically achieved through two methods: adsorption via physicochemical interactions, or encapsulation using polymer coatings.
The three-dimensional structure of Ta facilitates drug transport and release. Compared to dense bulk metals, porous metals are more conducive to drug retention [20,43]. Sautet et al. [20] first achieved the loading and gradual release of antibiotics from porous Ta cylinders. In vitro immersion results demonstrated that a 1 cm³ Ta cylinder could absorb and release at least 20 mg from 1 g of vancomycin. Such properties of porous Ta may aid in preventing or treating prosthetic joint infections. While convenient and simple, this method suffers from limitations such as restricted drug loading capacity and short release duration. Garbuz et al. [44] designed a porous Ta scaffold for local delivery of alendronate by immersing calcium-coated porous Ta in alendronate buffer solution and fixing it at room temperature for 7 days. In vivo experiments demonstrated that locally released alendronate inhibited osteoclast activity while enhancing osteoblast activity, resulting in increased new bone formation on the scaffold surface and promoting implant osseointegration.
Biopolymers exhibit biodegradability and biocompatibility in physiological environments, making them suitable as matrices for coating and delivering drugs onto porous Ta, thereby enabling effective control of drug release. Guo et al. [21] encapsulated the anticancer drug doxorubicin within a multilayer copolymeric film on porous Ta implants by electrostatically self-assembling hyaluronic acid, methylated collagen, and a terpolymer onto the implant surface, endowing the porous Ta with sustained drug delivery capability. Experiments demonstrated effective doxorubicin release for up to one month, effectively inhibiting proliferation of the chondrosarcoma cell line SW1353. This functionalized implant serves as a localized, sustained-release drug delivery system for reconstructive surgical treatment of bone tumors. Mckenzie et al. [45] loaded zoledronic acid into porous Ta. Six weeks after implantation in canine femurs, drug concentrations were 732.6 ng/g and 5.8–7.0 ng/g on the implant side and non-implant side, respectively, decreasing to 377.2 ng/g and 1.9–7.1 ng/g after 52 weeks. This demonstrates that porous Ta not only maintains local drug concentrations but also effectively controls systemic drug levels at low concentrations, which is crucial for preventing systemic toxicity. Biodegradable gelatin nanoparticles (GNPs), a type of peptide polymer, are commonly used as effective drug delivery carriers for controlled and targeted release. Zhao et al. [46] loaded GNPs hydrogel into porous Ta prepared via SLM to construct extracellular matrix microarchitecture. Endothelial cells derived from bone marrow mesenchymal stem cells were seeded onto GNPs-loaded porous Ta scaffolds and implanted into the subcutaneous tissue of nude mice. Histomorphometric analysis at 28 days post-implantation revealed that the scaffold significantly promoted capillary network formation.
Thus, porous Ta implants rely on their surface-open microporous morphology and internally interconnected three-dimensional structure to load various drugs and cytokines. Through surface functionalization, they achieve initial stability and long-term osseointegration for implant repair in systemic chronic diseases, demonstrating great potential for local drug delivery in artificial implants. However, constructing suitable drug carriers, maintaining the bioactivity and stability of drug-loaded coatings in vivo, and controlling the release rate of drugs and active factors remain major challenges.
1.6 Antimicrobial Coatings
Porous materials themselves can easily become carriers for bacterial colonization, increasing the risk of infection. Peri-implantitis caused by bacterial infection necessitates secondary surgical revision, imposing physical and psychological burdens on patients. Therefore, developing antimicrobial-functionalized bone defect repair materials is of great significance. Currently, few studies have reported antibacterial modification of AM porous Ta. On one hand, most research remains controversial regarding whether Ta inherently possesses antibacterial properties. It is argued that Ta's chemically stable nature and tendency to form a Ta₂O₅ oxide layer on its surface prevent the dissociation of Ta⁵⁺ even in vivo, thus lacking antibacterial activity [47]. Other studies suggest that the antibacterial activity of porous Ta is related to the surface-formed Ta₂O₅ layer, with amorphous Ta₂O₅ exhibiting excellent antibacterial properties, though the underlying mechanism remains unclear [48-49]. On the other hand, AM porous metals possess larger specific surface areas and more complex internal structures compared to traditional two-dimensional materials. Loading antibiotics via sustained-release coatings is complex and challenging to control their in vivo release kinetics.
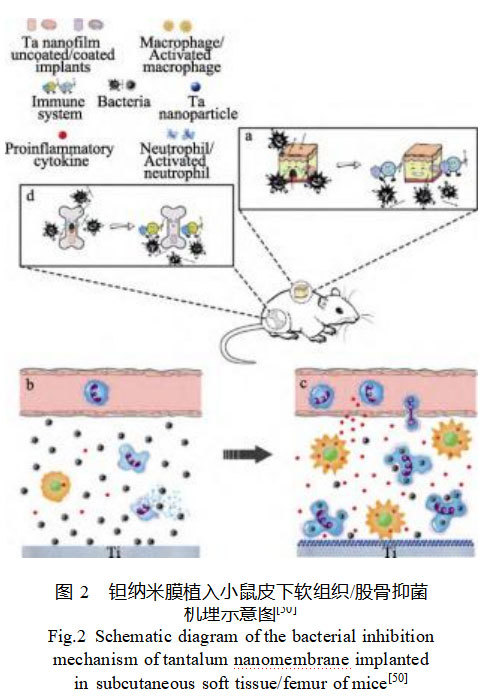
Ta nanomembranes have been demonstrated to exhibit antibacterial activity and inhibit bone resorption through endogenous immune modulation, with their antibacterial mechanism illustrated in Figure 2 [50]. Ta nanomembranes enhance neutrophil phagocytosis of bacteria, reduce neutrophil lysis, and promote macrophage release of pro-inflammatory cytokines. This immunomodulatory effect aids the host in eliminating bacteria, offering a safer and more effective alternative to traditional antimicrobial coatings that rely on toxic mechanisms to kill bacteria. Directly fabricating functionally integrated, topologically structured porous Ta orthopedic implants with nanoscale morphology using AM technology represents a simple and economical potential antimicrobial strategy.
2 Topological Structure Optimization
An ideal bone repair scaffold must possess at least three essential characteristics: 1) excellent biocompatibility; 2) mechanical properties matching those of natural bone tissue; and 3) a highly interconnected porous structure that facilitates cell adhesion, proliferation, and differentiation while allowing the transport of nutrients and metabolic waste [51]. The topological structure of AM porous Ta is closely related to its mechanical, physicochemical, and biological properties. Furthermore, AM technology enables precise generation of uniform or gradient geometric features on scaffold surfaces. These topological modifications, manifested as micro- and nanoscale protrusions, pits, or grooves, enhance cell adhesion by promoting integrin aggregation to form focal adhesions and guide stem cell differentiation [52-53]. From a macroscopic perspective, factors such as the overall scaffold architecture, pore-forming units, pore connectivity, porosity, and pore size distribution collectively regulate the process of bone tissue ingrowth into the scaffold. At the microscopic level, protein adsorption and retention within material pores determine subsequent cell adhesion, while the micro- and nano-structures of the scaffold further influence cellular proliferation behavior and specific gene expression.
2.1 Pore Size
Porous scaffolds facilitate nutrient transport, metabolic waste removal, cell proliferation and differentiation, intercellular signaling, and capillary formation through diffusion and permeation of fluid media. Consequently, pore size is a critical parameter in designing porous implants. Research indicates that pore sizes within the range of 150–1000 μm promote bone ingrowth; pores larger than 300 μm allow the formation of vascularized bone tissue; pores between 50–150 μm lead to osteoid growth; while porous structures with pores smaller than 50 μm only form fibrous tissue [54]. Both excessively large and small pore sizes adversely affect bone repair capacity. Large pores increase the distance between bone tissue and the porous scaffold interface, hindering bone healing. Conversely, small pores induce stress shielding, causing modulus mismatch between the implant and host bone, leading to host bone resorption and implant failure. An appropriate pore size can approximate the stiffness of surrounding bone, enabling the implant to effectively transmit loads and mitigate stress shielding effects. Luo et al. [55] utilized SLM technology to fabricate porous Ta scaffolds with pore sizes of 100–200, 200–400, 400–600, and 600–800 μm. In vivo and in vitro studies demonstrated that the 400–600 μm porous Ta scaffold exhibited superior osteogenic and angiogenic differentiation potential, yielding the most effective bone repair outcomes. Wang et al. [56] found that porous Ti6Al4V scaffolds with a pore size of 1000 μm exhibited superior bone regeneration and angiogenic promotion capabilities in vivo compared to scaffolds with pore sizes of 800 and 900 μm.
2.2 Porosity
Porosity denotes the percentage of void space within a solid structure. For scaffolds, porosity is a key parameter governing localized mechanical properties and permeability [9]. Human cancellous bone exhibits porosities ranging from 30% to 95%, providing a benchmark for designing porous implant porosities. In vivo bone regeneration around porous implants involves the recruitment, migration, and vascularization of surrounding bone tissue cells [54]. Extensive in vitro and in vivo biological studies indicate that relatively high porosities, while promoting cell proliferation, bone ingrowth, and vascularization, also compromise the mechanical properties of porous implants. Gao et al. [57] fabricated porous Ta scaffolds with porosities of 65%, 75%, and 85% using SLM technology. They observed that both compressive strength and elastic modulus increased with porosity. Higher porosity enhances permeability, facilitating nutrient transport and metabolic waste removal, thereby further promoting bone ingrowth and vascularization. Wauthle et al. [8] first applied an AM porous Ta scaffold with 80% porosity in a rat transect bone defect model, demonstrating excellent in vivo osteogenic performance and strong interface bonding between the implant and bone. Cheng et al. [58] fabricated porous Ti6Al4V with porosities ranging from 15% to 70%. The results indicated that the content of early differentiation markers (alkaline phosphatase) decreased with increasing porosity, while the content of late differentiation markers (osteocalcin, osteoprotegerin, vascular endothelial growth factor, and osteopontin) increased with increasing porosity. It was suggested that high-porosity structures could stimulate osteogenic differentiation. However, the effect of porosity on osteogenesis exhibited an opposite trend in vivo, where low-porosity scaffolds demonstrated higher alkaline phosphatase activity and osteocalcin expression [59]. It should be noted that discrepancies between in vivo and in vitro results may arise because static cell culture methods in vitro cannot replicate the complex physiological environment in vivo.As evident, the design of scaffold porosity typically involves numerous complex and conflicting characteristics and requirements within the body. For instance, scaffold permeability and stiffness compete with each other; higher porosity is often achieved at the expense of lower mechanical strength. Therefore, to ensure the scaffold meets specific mass transfer requirements while maintaining suitable mechanical properties, a balance must be found between porosity and strength.
2.3 Pore Geometry
The geometric arrangement of pores forms a three-dimensional support structure that significantly influences the mechanical properties, osteogenic potential, and vascularization of porous implants. Based on pore geometry, porous implants can be categorized into reticular lattices, random lattices, or functionally graded lattices.
Lattice structures involve repeating unit cells in three-dimensional space to achieve desired dimensions, forming regular implant architectures. Traditional bone defect implants typically employ lattice structures, often featuring uniform, regular geometries. While these satisfy the biomechanical properties of certain cancellous bone types, they exhibit significant discrepancies with the skeletal structures and physiological functions of different human body regions. In current AM-fabricated porous Ta research, lattice structures primarily include rhombic dodecahedrons, diamond shapes, and simple cubes. Among these, rhombic and rhombic dodecahedral structures have demonstrated lower stiffness compared to others, yet cannot withstand equivalent compressive loads under identical conditions [60]. Additionally, the triple-periodic minimal surface (TMPS) unit cell structure, a subset of reticular lattices, exhibits zero curvature in all three dimensions. It features axisymmetric stiffness, high specific surface area, and pore connectivity, demonstrating superiority in both biological and mechanical properties [61-62].
Traditional chemical vapor deposition (CVD)-fabricated porous Ta structures predominantly adopt random lattice configurations. Computer modeling and mathematical algorithms can also generate random lattice structures in AM technology. Random lattices, also termed “irregular” or “trabecular” lattices, lack fixed repeating unit structures. They can mimic human trabecular bone structures using biomimetic principles, with mechanical properties emulating trabecular bone's stress response in the human body [63-64]. Wang et al. [56] used electron beam melting (EBM) to fabricate both regular and irregular Ti6Al4V structures. They found that irregular pore structures promoted better adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells (BMSCs) compared to regular pore structures. Additionally, scaffolds with larger pores favored the expression of angiogenesis-related genes. Twelve-week implantation results in rabbit tibiae demonstrated that irregular structures and larger pore sizes more effectively promote vascular formation and bone tissue ingrowth, facilitating repair of large-sized bone defects.
Human bone itself constitutes a functionally graded system. For instance, the structural transition from cortical to cancellous bone is progressive, with smooth variations in pore distribution determining gradual changes in mechanical properties. A scaffold exhibiting gradient variations in specific properties (e.g., material composition, pore characteristics) across its design space to achieve one or more functions is termed a functionally gradient scaffold [65]. Such scaffolds mitigate stress shielding effects when adapting to complex in vivo loads, deliver selective bioactivity, and accommodate macroscopic bone geometry changes.
It should be noted that due to inherent material differences, identical topological parameters yield varying outcomes across different materials. Although extensive research has explored optimizing biomedical titanium alloy topologies for biological performance, the influence of pore structure on the mechanical properties, biocompatibility, and osseointegration efficacy of porous Ta remains understudied. Establishing the relationship between AM porous Ta topology and biomechanics/osseointegration represents a crucial future research direction.
3. Summary and Outlook
In recent years, AM porous Ta has demonstrated superior osteogenic potential compared to traditional orthopedic implants in various animal bone defect models and clinical reports, showcasing excellent clinical application potential. Related reports indicate that patients with bone defects still face risks such as poor osseointegration, bacterial infection, and aseptic loosening. Integrating multiple surface modification techniques with topological optimization methods is key to endowing AM porous Ta with osseointegration capability, antibacterial properties, and angiogenic promotion to address these challenges.
AM porous Ta possesses a larger specific surface area and complex internal three-dimensional structure compared to traditional two-dimensional materials. On one hand, this endows it with certain advantages in surface modification applications. For instance, anodic oxidation and alkaline heat treatment can form biomimetic micro-nano gradient structures resembling human bone architecture, offering benefits such as high drug adsorption capacity and prolonged release duration in sustained-release drug delivery systems. However, its intricate three-dimensional architecture also limits the applicability of certain surface modification methods. For instance, anodization cannot uniformly form nanotube films on the porous Ta surface or within its pores. Similarly, some functionalized coatings fail to uniformly cover and penetrate the internal pores of porous Ta, making it difficult to achieve stable bonding with the substrate surface. Consequently, most research on surface modification techniques for porous Ta remains confined to in vitro experiments or short-term in vivo animal model studies. Surface modification techniques have been extensively applied to two-dimensional materials. As biomaterial scaffolds play an increasingly vital role in tissue engineering, it is imperative to extend the application of surface modification technologies into three-dimensional spaces. Future efforts should focus on further controlling surface modification conditions and optimizing process parameters to fully leverage the performance and structural advantages of porous Ta. This will enable the development of multifunctional bone defect repair scaffolds tailored to specific patient needs.
The development of local drug delivery systems offers an innovative and effective direction for AM porous Ta in orthopedic applications, though exploration in this area remains relatively scarce. It remains unclear whether various antibiotics or anticancer drugs may affect the performance of AM porous Ta and consequently influence osseointegration. Future research should focus on the synergistic effects and overall properties between AM implants and their drug delivery systems. Currently, loading of multiple antimicrobial and antitumor drugs has been achieved on titanium implants, but studies using Ta as a carrier are scarce, likely due to Ta's high cost and processing challenges. AM porous titanium coated with Ta holds promise for addressing this issue. However, whether it possesses comparable biocompatibility and osseointegration properties to AM porous Ta, along with the in vivo coating adhesion strength, remains to be investigated.
AM technology's capabilities in personalized design and pore characteristic control make topological optimization another pathway for functionalizing orthopedic implants. Its interconnected pore structure provides space for bone tissue ingrowth and vascularization processes. Pore volume fraction, pore size, and pore geometry are critical structural parameters for AM porous Ta. When appropriately selected, these parameters promote vascularization and bone tissue ingrowth in porous Ta implants, forming biological interlocking with the host to ensure long-term in vivo stability. However, optimal structural parameters remain undetermined. The authors believe developing functionally graded materials to meet specific requirements should be a future research focus. Furthermore, integrating Finite Element Analysis (FEA) and Topological Optimization (TO) can further refine structural and 3D printing parameters to establish the relationship between AM porous Ta topology and mechanical properties, in vitro biology, and in vivo bone ingrowth.
Currently, most research on surface modification and topological optimization of AM porous Ta focuses on in vitro biological experiments and small animal bone defect models, presenting a significant gap from clinical application. The safety and efficacy of such functionalized AM porous Ta implants in vivo require rigorous, long-term evaluation, representing a substantial challenge for researchers and surgeons.
References: Article ID: 1001-3660(2023)07-0001-10
DOI: 10.16490/j.cnki.issn.1001-3660.2023.07.001
Research Progress on Surface Modification and Functionalization of 3D-Printed Porous Tantalum
Stardust Technology (Guangdong) Co., Ltd. is a leading domestic enterprise specializing in the mass production of medical-grade spherical tantalum powder using radiofrequency plasma spheronization technology. Characterized by high purity, low oxygen content, excellent sphericity, and superior flowability, this spherical tantalum powder is ideally suited for 3D printing precision medical implants such as bone screws, bone plates, and joint prostheses. The material's outstanding biocompatibility promotes bone healing. The company has participated in formulating multiple national standards for medical implants and assisted in over 500 clinical applications of tantalum orthopedic implants. For more information, please contact Cathie Zheng at +86 13318326187.

