High-temperature oxidation behavior of Si-Cr-Ti coating on Nb521 surface
Release time:
2025-07-15
Niobium alloys have been widely used in the aerospace and nuclear industries due to their high melting point, high hardness, excellent mechanical properties and corrosion resistance [1]. However, due to the low initial oxidation temperature of niobium, the oxidation resistance of niobium and niobium alloys is relatively poor. Vilasi [2] reported that pure niobium will undergo severe oxidation at 600 °C. Therefore, the oxidation resistance of niobium alloys is usually improved by coating silicide coatings, oxide coatings or precious metal coatings on the surface of niobium alloys [3]. Among them, the Si-Cr-Ti coating system has the advantages of strong high-temperature self-repairing ability, good oxidation resistance, low price and simple preparation process, and has become a commonly used coating system for niobium alloys. The Si-Cr-Ti coating system has been successfully applied to the American R-1E (110 N), R-4D (490 N), R-6C (22 N) engines and the Apollo spacecraft. Sylviania uses R512E (Si-20Cr-5Ti) silicide coating as a protective coating for niobium alloy nozzles. The coating performance is very stable in air at 1400 °C [4-5]. However, with the development of aerospace technology, the requirements for the operating temperature and service life of niobium alloy hot end components are getting higher and higher, and new requirements are put forward for coating performance. Therefore, a series of studies on Si-Cr-Ti coating modification have been carried out at home and abroad. Studies [6-11] show that the oxidation resistance of the coating can be improved by adding appropriate amounts of rare earth oxides or modifying elements (such as molybdenum, zirconium, germanium, etc.). Domestic aerospace materials research institute [6], Shanghai Silicate Research Institute [7-8], Central South University [9] and many other institutions have conducted research on Si-Cr-Ti coating system on niobium alloy surface. Wang Yu et al. [7] prepared a Si-Cr-Ti coating modified with germanium, molybdenum and tungsten on the surface of C-103 alloy, which failed after oxidation at 1600℃ for 17h; Zhai Jinkun et al. [10] prepared a Si-15Cr-10Ti-10Zr-0.5Y2O3 coating on the surface of C-103 alloy, which increased in mass by 1.84mg/cm2 after oxidation at 1400℃ for 4.5h. At present, domestic and foreign literature focuses on the reports on the effects of modifying elements on coating performance, and there are few reports on the existence form, action mechanism and high-temperature oxidation behavior of modifying elements.In this work, tungsten and Al+Y2O3 modified Si-Cr-Ti coatings were prepared on the surface of niobium alloy by slurry sintering method. The oxidation behavior of these two coatings at 1400 ℃ and the existence form and action mechanism of the modified elements were studied, and the oxidation mechanism of the coating was discussed.
1 Experiment
1.1 Preparation of samples
The experiment used Nb521 alloy, silicon powder (99.5%), chromium powder (99.5%), titanium powder (99%), tungsten powder (99%), aluminum powder (99%), and Y2O3 powder (99.9%) as the main raw materials. Among them, the main components (mass fraction) of the Nb521 alloy used are: wMo5%, wW2%, wZr1%, and the balance is niobium. Firstly, the Nb521 alloy was processed into a sample with a size of 10mm×10mm×1mm by a wire cutting machine. The sample was polished with sandpaper, ultrasonically washed with alkali and acid, and washed with distilled water, and then dried. Then, the powdered raw materials were weighed according to a certain ratio, an appropriate amount of binder was added, and alcohol was used as a solvent. The coating slurry was prepared by high-energy ball milling for 24 hours under the protection of argon atmosphere. Finally, the slurry was sprayed on the surface of the niobium alloy sample and dried at 80℃ for 1 hour. Then, it was placed in a vacuum sintering furnace and kept at 1420℃ for 0.5h. Then, it was cooled to room temperature with the furnace to obtain the sample. The heating rate during the sintering process was 10℃/min.
1.2 Performance testing and analysis
The static oxidation behavior and discontinuous oxidation behavior of the sample were tested in a high temperature furnace at 1400 ℃ in an air atmosphere; the oxidation weight gain of the sample was measured using an electronic analytical balance with an accuracy of 0.1 mg and a maximum range of 220 g; the surface structure morphology of the coating material was observed using a Sirion 200 scanning electron microscope (SEM) produced by the Dutch FEI company; the cross-sectional morphology of the coating was observed using a Japanese electron probe microanalyzer (EPMA) equipped with a spectrometer (WDS), and the composition of the cross-sectional structure of the coating was analyzed.
2 Results and discussion
2.1 Surface morphology of the coating before and after oxidation
Figure 1 shows the SEM morphology of the surface of the Si-Cr-Ti-W coating before and after oxidation. As shown in Figure 1 (a), the surface of the coating before oxidation is relatively rough, with island-like protrusions. EPMA composition analysis shows that the tungsten content here is significantly higher than that of the main body of the coating. Wang Yu et al. [7] believed that the protrusions were due to the high melting point of tungsten (3380 °C), which still existed in the solid state during the slurry sintering process. During the cooling process, the unmelted tungsten particles served as liquid phase nucleation centers, gradually forming larger particles and remaining on the coating surface in the form of islands. When the area around the island-like protrusions was enlarged and observed, a small number of microcracks were observed. This was mainly due to the mismatch in thermal expansion coefficients between the tungsten-rich island-like protrusions and the main body of the coating. During the cooling process, stress concentration was prone to occur in the area around the island-like protrusions. As shown in Figure 1 (b), the surface morphology of the Si-Cr-Ti-W coating changed significantly after oxidation. The surface was covered with a uniform molten glass film, and the microcracks were reduced, but the island-like protrusions still existed. The glass film is generated by the reaction of silicon and oxygen in the coating at high temperature. The main component is SiO2, which has a certain fluidity. Under the action of surface tension, SiO2 can effectively fill the cracks and holes in the coating, and significantly improve the density of the coating surface. Since the diffusion rate of oxygen ions in the molten SiO2 glass film is very low, the oxidation resistance of the alloy is improved.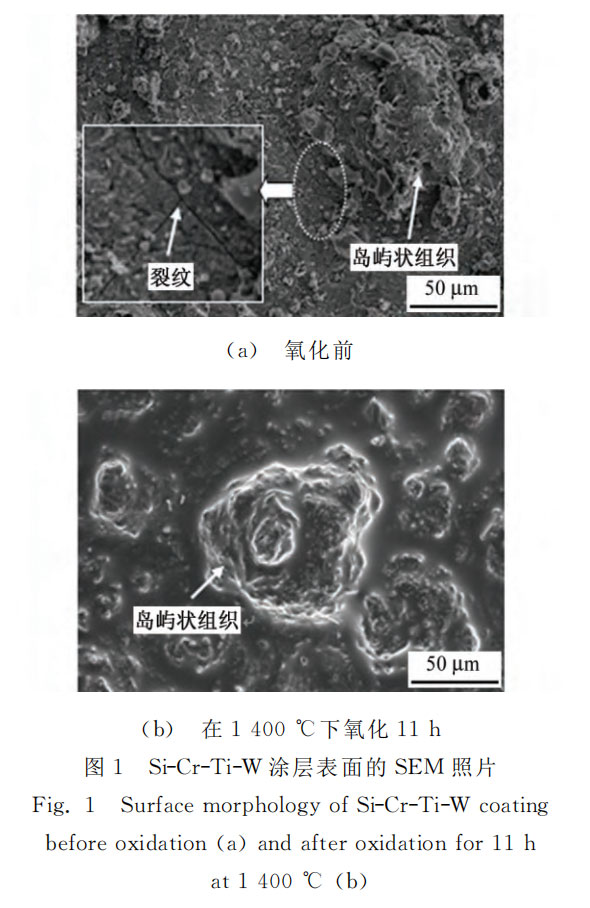
As shown in Figure 2, the particles of the coating before oxidation are relatively small, the surface is relatively smooth and the density is high. Compared with Si-Cr-Ti-W coating, the surface of Si-Cr-Ti-Al-Y2O3 coating after oxidation is more compact. This is mainly because the silicon and aluminum in the coating are oxidized to SiO2 and Al2O3 at high temperature. The composite glass film formed is denser than the pure SiO2 glass film. The composite glass film has high thermal enthalpy, strong high temperature stability and moderate thermal expansion coefficient, which reduces the initiation of defects during oxidation [12].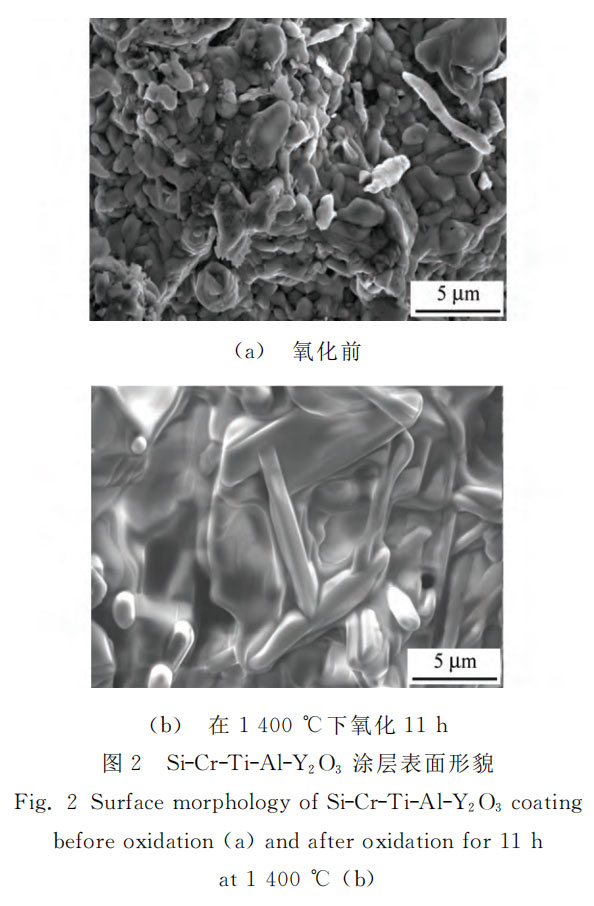
2.2 Cross-sectional morphology and composition of coating before and after oxidation
2.2.1 Si-Cr-Ti-W coating
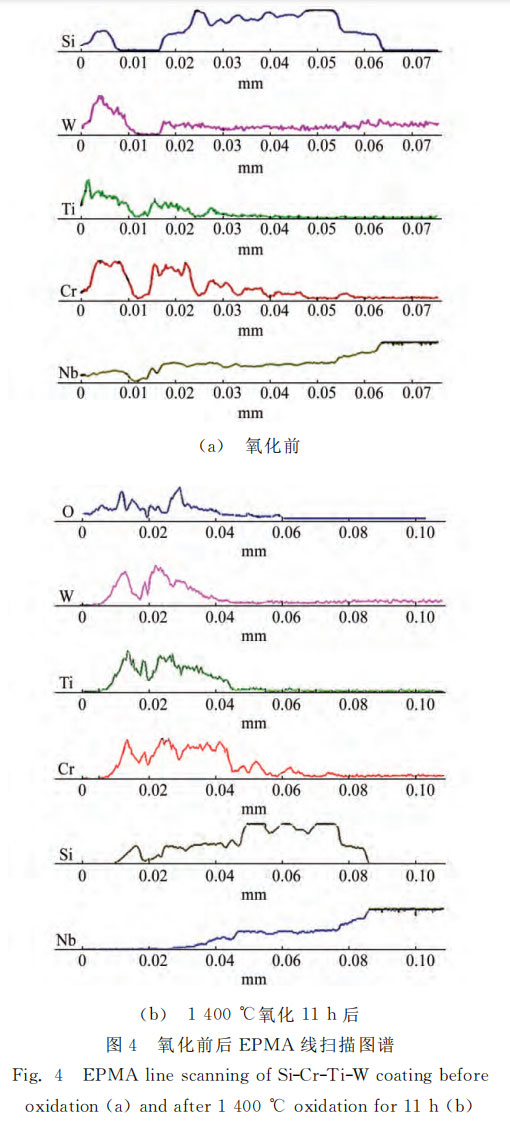
Figures 3 and 4 show the cross-sectional morphology and EPMA line scan spectrum of Si-Cr-Ti-W coating before and after oxidation. As can be seen from Figure 3 (a), the coating is uniform and dense as a whole, well bonded to the substrate, with a thickness of about 50 μm. It can be roughly divided into three layers, namely the outer layer, the main layer and the diffusion layer. The outer layer of the coating is about 10 μm thick, and its main components are MSi2 and a small amount of M5Si3 (M represents chromium, titanium, tungsten and niobium). The tungsten content is relatively high, which is mainly due to the migration of tungsten to the surface during the sintering process [7]. As can be seen from the figure, the layer is relatively loose, with holes and easy to peel off. This may be because: (1) During the sintering process, the silicon in the coating diffuses rapidly into the substrate, the silicon content on the surface decreases, and the diffusion rate of refractory elements such as chromium, titanium, and tungsten is relatively low [13], and they remain on the surface to form a loose surface layer; (2) The diffusion of silicon atoms requires the vacancies in the silicon sublattice to move in the opposite direction or move along the grain boundary. A large number of vacancies aggregate to form Kirkendall holes [14].
The main layer of the coating is about 30μm thick, and the main component is MSi2, in which the niobium content is relatively high, and the chromium, titanium, and tungsten contents in the main layer close to the outer layer are higher than those close to the substrate. This is because the slurry adjacent to the substrate diffuses with the substrate during the sintering process, which causes a slight decrease in the content of chromium, titanium, and tungsten elements near the substrate. During the sintering process, chromium, titanium, tungsten in the coating and niobium in the substrate react with silicon to generate MSi2, which constitutes the main layer of the coating. The diffusion layer of the coating is about 8μm thick and its main component is Nb5Si3. During the sintering process, the niobium element in the substrate and the silicon element in the coating diffuse with each other and react to generate Nb5Si3 low-silicide. The Nb5Si3 diffusion layer provides a composition gradient with a gradually changing silicon content, so that the thermal expansion coefficient can transition smoothly between the substrate and the coating, reducing the generation of cracks [15-16] and improving the thermal shock resistance of the coating. As shown in Figure 3 (b), the coating can still be divided into the outer layer, the main layer and the diffusion layer after oxidation, but its thickness has changed significantly compared with that before oxidation. The outer layer of the coating becomes uneven due to oxidation, with more holes, and the thickness increases from 10μm to about 21μm. Its composition changes to SiO2 and a small amount of Cr2O3, TiO2, WO2, Nb2O5, etc. The thickness of the main layer of the coating decreases from 30μm to 23μm, and the composition is still mainly MSi2. The thickness of the diffusion layer increases from 8μm to about 12μm, and the composition is still mainly Nb5Si3. During the high-temperature oxidation process of Si-Cr-Ti-W coating, silicon, chromium, titanium, tungsten, niobium and other elements react with oxygen elements in the outer layer to generate SiO2, Cr2O3, TiO2, WO2, Nb2O5, etc. The surface element content decreases, and silicon, chromium, titanium, and tungsten in the main layer diffuse to the outer layer along the concentration gradient to participate in the oxidation reaction. As this process proceeds, silicon, chromium, titanium, and tungsten elements in the main layer are gradually consumed, and the main components gradually change from MSi2 to NbSi2, and then to Nb5Si3, so the thickness of the main layer decreases and the thickness of the diffusion layer increases. When the MSi2 in the main layer is completely exhausted and converted into Nb5Si3, the coating cannot provide silicon elements to react with oxygen elements in time. The surface defects cannot be repaired in time due to the lack of SiO2. Oxygen elements enter the substrate through the defects, causing severe oxidation, and the coating loses its protective effect.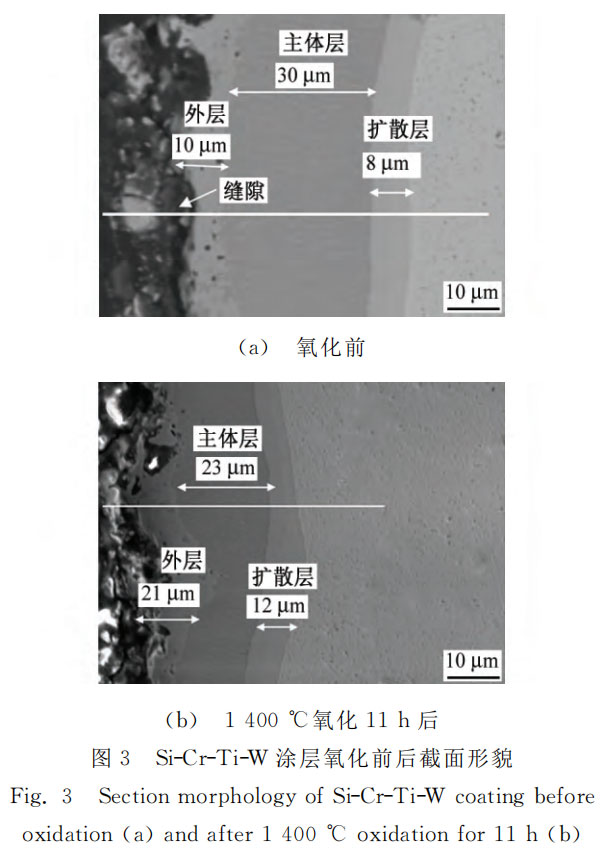
 2.2.2 Si-Cr-Ti-Al-Y2O3 coating
2.2.2 Si-Cr-Ti-Al-Y2O3 coating
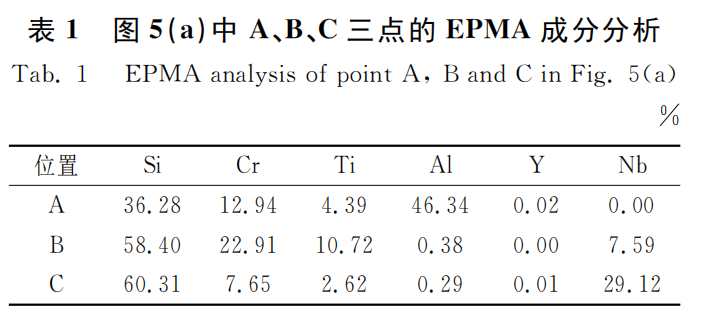
Figures 5 and 6 show the cross-sectional morphology and component line scanning spectra of the Si-Cr-Ti-Al-Y2O3 coating before and after oxidation. Table 1 shows the spectral component analysis results of A, B, and C in Figure 5 (a). As can be seen from Figure 5 (a), the Si-Cr-Ti-Al-Y2O3 coating is divided into three layers, namely the outer layer, the main layer, and the diffusion layer. Among them, the outer layer is black in the figure, and its color is similar to phenolic resin. EPMA is performed on A, and it is known that this layer is composed of a large amount of aluminum and a small amount of (Cr, Ti) Si2, with a thickness of about 15μm. The thickness of the main layer is about 60μm, mainly composed of (Cr, Ti) Si2 and Nb-Si2. The gray B area in Figure 5 is mainly (Cr, Ti) Si2, and the light gray C area in Figure 5 is mainly NbSi2. The thickness of the diffusion layer is about 11μm, and the main component is Nb5Si3. As shown in Figure 5, the surface of the coating becomes uneven after oxidation, and the diffusion layer increases from 11μm to 20μm. The oxidation process of Si-Cr-Ti-Al-Y2O3 coating is similar to that of Si-Cr-Ti-W coating. The difference is that the aluminum and silicon in the outer layer react with oxygen at high temperature to form a composite oxide film whose main components are Al2O3 and SiO2 [17]. The composite oxide film is dense and has strong high-temperature stability, which hinders the diffusion of oxygen elements.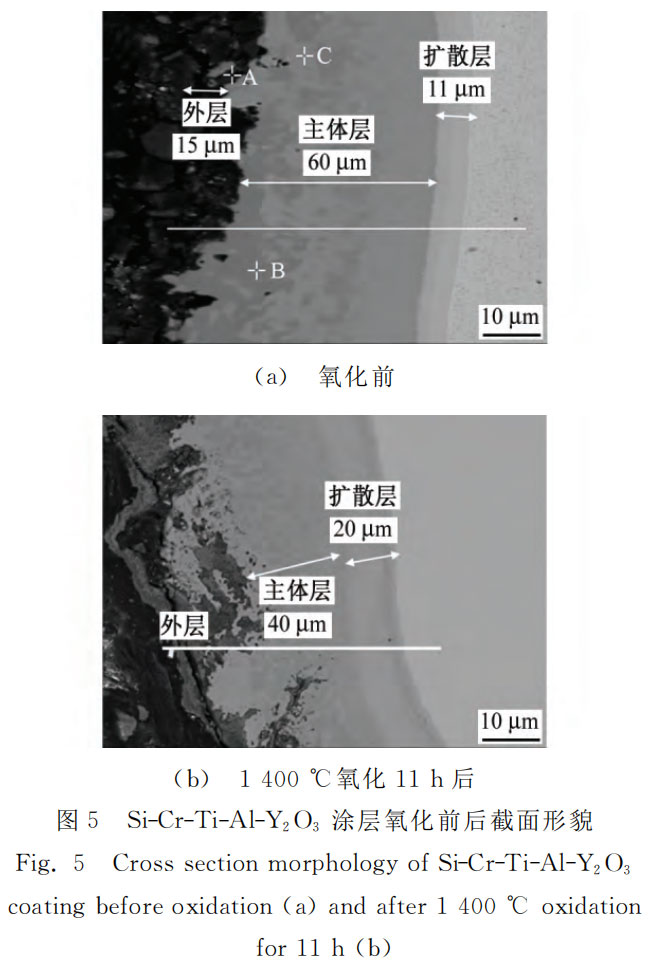
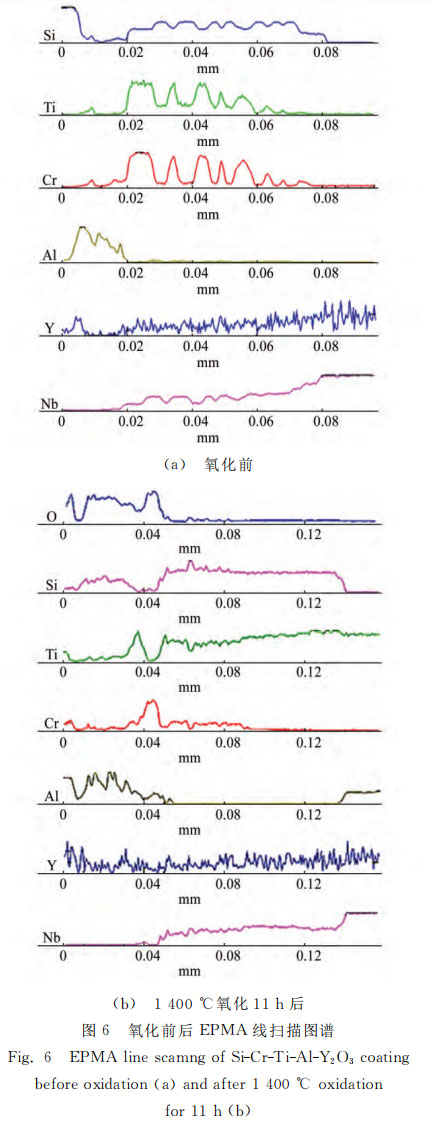
2.3 Oxidation kinetics of coating
According to Wagner oxidation kinetics theory, the oxidation rate of metal is controlled by the diffusion of positive and negative ions through the formed oxide film. Δm represents the weight gain per unit area per unit time, t represents time, and Kp represents the oxidation rate constant. Assuming that t=0, Δm=0, then we have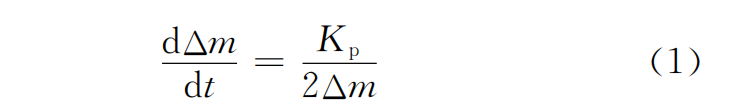
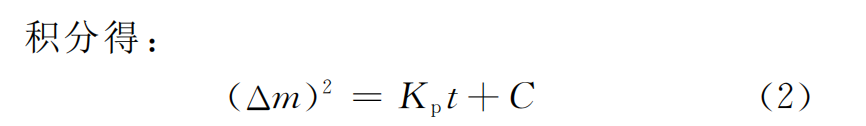
The premise of this kinetic model is that the temperature is continuously stable at any time during the oxidation process. However, in actual oxidation, due to problems such as heat transfer and surface air flow [18-19], the temperature in different regions is uneven. Therefore, Daniel et al. [20] proposed to modify the model as follows. Assuming that when the sample t→ti, Δm→Δmi, we have
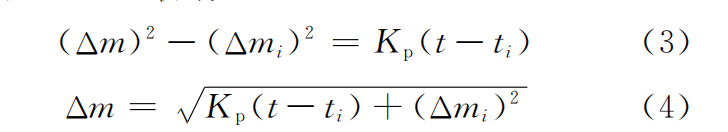
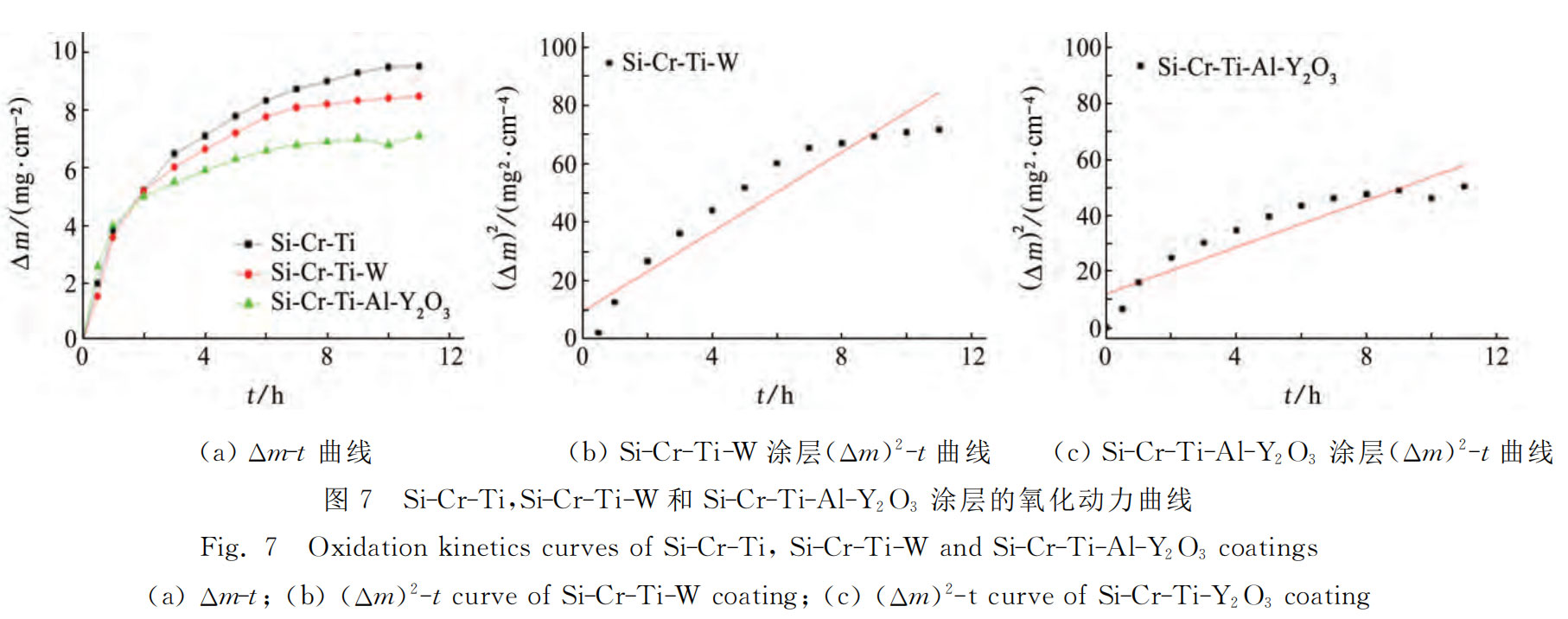
The Δm-t relationship curve and (Δm)2-t relationship curve of Si-Cr-Ti coating, Si-Cr-Ti-W coating and Si-Cr-Ti-Al-Y2O3 coating at 1400℃ are shown in Figure 7. As can be seen from Figure 7 (a), oxidation can be divided into three stages. The weight gain is fast in the initial oxidation stage (0-1h). At this time, silicon and aluminum in the coating react with oxygen to generate SiO2 and Al2O3, forming a molten glass film, but it has not yet completely covered the surface. The weight gain rate slows down in the middle oxidation stage (1-7h). At this time, the molten glass film completely covers the substrate and continues to thicken with time, significantly reducing the diffusion rate of oxygen elements, and the weight gain of the coating slows down. In the late oxidation stage (7-11h), due to the thick molten glass film, the diffusion rate of oxygen elements is reduced, the silicon consumption in the coating is reduced, and the oxidation weight gain tends to be stable. The weight gain per unit area of the three coatings is not much different within 2h, but after 2h, the weight gain rate of the coating with added modified elements is significantly lower than that of the original coating. After oxidation at 1400℃ for 11h, the weight of Si-Cr-Ti coating increased by 9.56mg·cm-2, that of Si-Cr-Ti-W coating increased by 8.34mg·cm-2, and that of Si-Cr-Ti-Al-Y2O3 coating increased by 6.96mg·cm-2. It can be seen that adding tungsten, aluminum and Y2O3 to Si-Cr-Ti coating can improve the oxidation resistance of coating. This is because: (1) tungsten reacts with silicon to generate WSi2 during sintering process, and aluminum and Y2O3 can also generate WSi2.[14, 21] believed that WSi2 reacts with oxygen to generate SiO2 and W5Si3, and the diffusion rate of oxygen in SiO2 and W5Si3 is low; (2) During the sintering process, the aluminum in the coating has a low melting point and high activity, and the liquid phase formed is conducive to sintering, reducing the generation of surface defects and the volatilization of silicon; (3) Y2O3 particles are distributed in the coating matrix and can serve as nucleation centers during the sintering process, which is conducive to the refinement of grains and the densification of the coating, reducing the formation of defects such as holes and cracks, improving the surface quality of the coating, and thus improving the oxidation resistance [6]. It can also be seen from Figure 7 (a) that the effect of adding aluminum and Y2O3 is better than that of tungsten. This is because aluminum and silicon react with oxygen to form a composite oxide film, which is denser than the pure SiO2 oxide film of the Si-Cr-Ti-W coating. The composite glass film has high thermal enthalpy, strong high temperature stability and moderate thermal expansion coefficient, so the coating has higher oxidation resistance than the Si-Cr-Ti-W coating. Figure 7 (b) and Figure 7 (c) show that (Δm)2 and t are basically linear, which shows that the oxidation curves of the two modified coatings at 1400 °C follow the parabolic law. By fitting the oxidation data, the Kp values of Si-Cr-Ti-W coating and Si-Cr-Ti-Y2O3 coating are 6.79mg2·cm-4·h-1 and 4.18mg2·cm-4·h-1, respectively. Substituting them into formula (4), the kinetic equations of the weight gain of the two coatings at 1400℃ constant temperature oxidation are shown in formula (5) and (6):
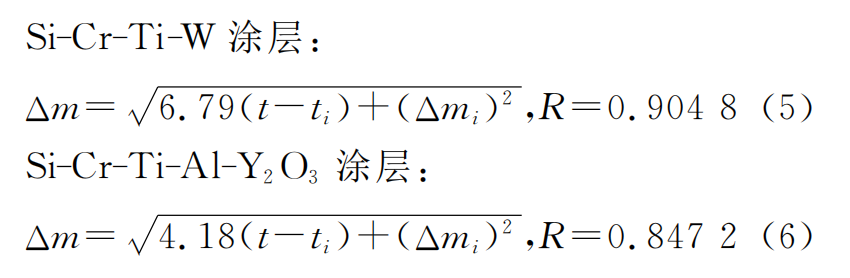
 3 Conclusion
3 Conclusion
(1) The oxidation process of Si-Cr-Ti-W coating at 1400℃ follows the parabolic law, and the kinetic equation is Δm = 6.79 (t-ti) + (Δmi) 2. The melting point of tungsten is relatively high. It will migrate to the outer surface during the sintering process and serve as a nucleation center during the cooling process to form an island-like structure. During the oxidation process, WS12 reacts with oxygen to generate SiO2 and W5Si3. The diffusion rate of oxygen in SiO2 and W5Si3 is relatively low, which improves the oxidation resistance of the coating to a certain extent.
(2) The oxidation process of Si-Cr-Ti-Al-Y2O3 coating at 1400 ℃ follows the parabolic law. The kinetic equation is Δm = 4.18 (t-ti) + (Δmi) 2. The addition of Y2O3 is beneficial to improve the surface quality of the coating. Aluminum has a low melting point and high activity. The liquid phase formed is conducive to sintering, reducing the generation of surface defects and the volatilization of silicon. During the oxidation process, the reaction generates Al2O3 and SiO2 composite oxide films. The composite oxide film is uniform and dense, has strong high-temperature stability and a moderate thermal expansion coefficient, which significantly improves the oxidation resistance of the coating.
Paper citation information
Corrosion and Protection Vol. 37 No. 5 May 2016
Niobium tungsten alloy Nb521 material has excellent room temperature and high temperature mechanical properties and the advantages of high melting point, low density, high temperature and high strength and good machinability. The spherical Nb521 alloy powder produced by Stardust Technology is made by radio frequency plasma spheroidization process, which has the characteristics of high purity and low oxygen, high sphericity, smooth surface, no satellite, uniform particle size distribution, excellent flowability, high bulk density and vibration density. It is widely used in the manufacture of aerospace engines, weapon thrusters, rocket missile liquid bi-component engines, nuclear reactors, submersibles, gas turbines, automobile engines, diesel engines, high-temperature furnace heating belts, high-temperature molds, high-temperature fixtures, and high-temperature crucibles.
http://en.stardusttech.cn/products_det/179.html
For more details, please contact Manager Vicky at +86-13318326185


