Microstructure and Mechanical Properties of Refractory High-Entropy Alloys WTaCrVTi Prepared by the MA-SPS Process
Release time:
2025-09-08
1 Introduction
Tungsten is considered one of the candidate materials for high-temperature plasma environments in nuclear fusion reactors due to its advantages of high density, high melting point, high strength, high hardness, high thermal conductivity, low physical sputtering rate, and low hydrogen and isotope retention rates [1-4]. To further enhance tungsten's performance, researchers have developed a series of high-performance tungsten alloys and tungsten-based composites [5-10], yet these still fall short of fully meeting fusion reactor requirements.
High-entropy alloys (HEAs), composed of multiple elements mixed in equimolar or near-equimolar ratios, exhibit superior properties unmatched by traditional alloys, such as high strength, high hardness, high wear resistance, and excellent stability [11-14]. In 2010, Senkov et al. [15] first employed vacuum arc melting to synthesize NbMoTaW-containing refractory high-entropy alloys (RHEAs), achieving a microhardness of 4455±185 MPa (454±19 HV)—exceeding any pure element material. Subsequently, researchers developed a series of tungsten-containing RHEAs incorporating Group IV (Ti, Zr, Hf), Group V (V, Nb, Ta), Group VI (Cr, Mo, W), and non-refractory elements such as Al, Si, Co, and Ni. Currently, most studied tungsten-containing RHEAs incorporate Nb or Mo. However, Nb and Mo exhibit long-lived radioactivity after neutron irradiation in nuclear fusion reactors [16-17], complicating subsequent maintenance and disposal. In 2019, El-Atwani et al. [18] employed magnetron sputtering to fabricate quaternary nanocrystalline WTaCrV alloys. Irradiation with 3 MeV Cu⁺ ions was conducted at both room temperature and 1050 K. No radiation-induced dislocation loops were observed in the irradiated samples, demonstrating excellent radiation resistance. In 2017, Waseem prepared WxTaCrVTi (x = 32at%–90at%) [19] and WTaCrVTix (x = 0.4at%, 7at%) [20] alloys with varying atomic ratios using mechanical powder mixing and discharge plasma sintering. Among these, W₀.₄₂TaCrVTi and WTaCrVTi₇ exhibited outstanding strength and hardness values of 2314 and 2069 MPa, and 793 and 714 HV, respectively. In 2021, Kim et al. [21] prepared an equimolar WTaCrVTi high-entropy alloy via arc melting. This alloy exhibited a room-temperature compressive strength of 1588±128 MPa with a strain of 23.4%±5.7%, and a compressive strength of 1210±43 MPa at 1073 K. The elements W, Ta, V, Cr, and Ti possess low neutron activation characteristics, making them suitable for neutron-irradiated environments in nuclear fusion reactors. Additionally, Ta exhibits a high melting point, excellent mechanical properties, and radiation resistance [22]; V enhances the strength and hardness of HEAs [15]; Cr provides outstanding high-temperature oxidation resistance [23]; and a small amount of Ti reduces the abundance of Cr-rich and V-rich phases in the alloy, improving microstructural uniformity [20]. Therefore, the novel WTaCrVTi RHEA alloy shows promise for nuclear fusion reactors. Currently, research on WTaCrVTi alloys remains scarce. Alloy preparation typically employs magnetron sputtering, arc melting, or mechanical mixing of powders followed by sintering. However, these methods yield microstructures with inhomogeneities, leading to element segregation and enrichment, which hinders the formation of high-entropy alloy structures.
Mechanical alloying (MA) offers advantages of simplicity and low cost. Typically conducted in high-energy ball mills, the process subjects alloy powders to repeated impacts, cold welding, and fragmentation. This not only promotes atomic diffusion within the powder, achieving atomic-level alloying of elements, but also refines the powder grain size.
Spark plasma sintering (SPS) rapidly sinter powders into high-density bulk materials using large pulsed currents combined with axial pressure. SPS offers advantages such as rapid heating and cooling rates, low sintering temperatures, short holding times, controllable grain size, and grain boundary regulation, enabling the production of materials with superior properties [24].
To enhance the homogeneity of elements within the alloy and promote the formation of a high-entropy alloy microstructure, this work employs MA combined with SPS to prepare WTaCrVTi high-entropy alloys. The study investigates the effects of ball milling time on the morphology, particle size, and element distribution of the alloy powder, as well as the phase composition and microstructure of the sintered alloy. The mechanical properties of the alloy are also characterized. This provides an experimental foundation for the preparation and performance optimization of tungsten-containing RHEAs.
2. Experimental
Raw materials included W powder (purity 99.98%, 1–5 μm), Ta powder (purity 99.9%, ~45 μm), Cr powder (purity 99.5%, ≥45 μm), V powder (purity 99.5%, ≥45 μm), and Ti powder (purity 99.99%, ≥48 μm). Powders were weighed according to the W₂₃.₅Ta₂₃.₅Cr₂₃.₅V₂₃.₅Ti₆ ratio and loaded into 500 mL stainless steel ball mills. To prevent cold welding during ball milling, 5 wt% ethanol was added as a process control agent. 10 mm and 6 mm diameters as grinding media, with a size ratio of 1:3 and a ball-to-powder mass ratio of 15:1. The milling jar was evacuated and purged with argon to 0.05 MPa. Mechanical alloying was conducted on a QM-3SP4 planetary ball mill (Nanjing Nanda Instrument Co., Ltd.) at 250 rpm, with grinding durations of 1, 5, 10, 20, and 40 hours. If grinding exceeded 40 hours, severe agglomeration of alloy powder particles occurred, with significant adhesion to the grinding balls and vessel walls, resulting in a powder yield below 50%. Additionally, the alloy powder exhibited excessively high surface activity, prone to spontaneous combustion. The ground powder was dried at 80°C in a vacuum oven for 10 hours.
The mechanically alloyed powder was sintered in a LABOX-1575 discharge plasma sintering furnace (Japan SinterLand). The temperature was raised at a rate of 100°C/min to 1500°C, held at 50 MPa pressure for 10 min, and then cooled with the furnace. The final cylindrical sintered specimens had a diameter of 20 mm and a thickness of 4 mm. For simplicity, the sintered alloys from powders with different ball milling durations are denoted as Ti6-xh (where x represents the ball milling duration in hours).
The top and bottom surfaces of the cylindrical specimens were sequentially polished using 600-grit and 800-grit diamond abrasive paper. Subsequently, the sintered samples were cut into cylindrical compression specimens with a diameter of 4 mm and a height of 6 mm using wire-cutting technology.
The phase composition of the materials was analyzed using an X-ray diffractometer (XRD, X'pertPRO, Netherlands) with a tube voltage of 40kV, tube current of 30mA, and a Cu-Kα radiation source. The microstructure and chemical composition were examined using a scanning electron microscope (SEM, Nova NanoSEM 450, Netherlands) , an energy dispersive spectrometer (EDS), and an electron probe microanalyzer (EPMA, JXA-8530FPLUS, Japan) were used to examine the microstructure and perform compositional analysis, respectively.
The compressive strength of the alloy was tested using a universal material tester (Zwick Z020, Germany). The alloy hardness was determined using a Vickers hardness tester (WilsonHardness, 430SVD, USA) with a load of 15 kg and a holding time of 10 s. The average value was taken after five measurements. The actual density ρ_a of the alloy bulk sample was measured using a ZY-300Z density balance based on the Archimedes displacement principle. The theoretical density ρtheor of RHEAs was calculated using the theoretical density formula (1) for disordered solid solutions:

where ρi is the density of the constituent element; Ai is the atomic mass of the constituent element; Ci is the content of the constituent element. The calculated theoretical density of the alloy was 12.4 g/cm³.
The relative density Rc of the alloy was calculated using formula (2):

3 Results and Discussion
3.1 Composition and Structure of Alloy Powders
Figure 1 shows the XRD patterns of the alloy powders after different ball milling durations. The XRD patterns of each powder group are similar, with diffraction peaks for W, Ta, Cr, V, and Ti elements observable. However, as the ball milling time increases, the intensities of the diffraction peaks for all elements decrease. Particularly for the powder milled for 40 hours, the intensities of the Cr and V diffraction peaks are significantly weakened, with W and Ta becoming the main diffraction peaks. Simultaneously, the diffraction peaks exhibit broadening and a shift toward higher angles. Based on the (110) crystal plane, according to Bragg's equations (3) and (4) and Scherrer's equation (5):
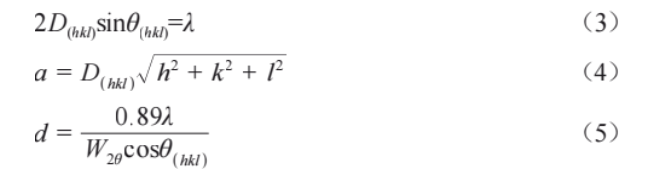
In the equation, D(hkl) represents the interplanar spacing, θ denotes the diffraction angle, λ is the wavelength of the X-ray diffraction beam (Cu target, 0.15406 nm), a is the lattice constant, d is the average grain size, and W2θ is the half-width at half-maximum.
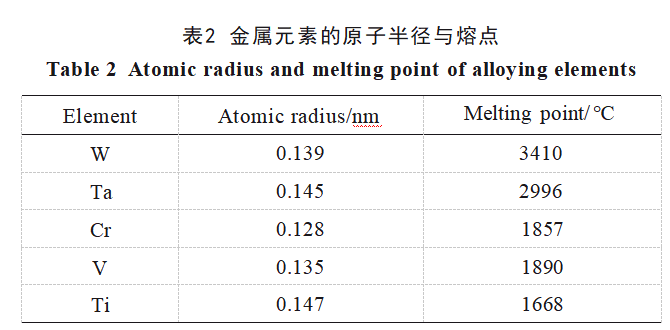
The average grain sizes, lattice constants, and lattice distortions of W and Ta after ball milling for 1 and 40 hours were calculated respectively, with the data shown in Table 1. As indicated in the table, the lattice constants of ball-milled W and Ta are both smaller than those of conventional W and Ta (0.3165 and 0.3303 nm, respectively), suggesting that other elements have solid-solved into W and Ta. The solid solution sequence of metallic elements depends on their melting points; generally, elements with lower melting points exhibit greater atomic diffusion propensity. Atomic radii and melting points of W, Ta, V, Cr, and Ti are listed in Table 2. In this alloy system, V, Cr, and Ti have significantly lower melting points than W and Ta. Additionally, Cr and V possess smaller atomic radii, making them readily diffusible into W and Ta. This diffusion causes lattice contraction and distortion in W and Ta. Consequently, as ball milling time increases, the XRD diffraction peak intensities corresponding to Cr and V diminish, while the W and Ta diffraction peaks shift toward higher angles.
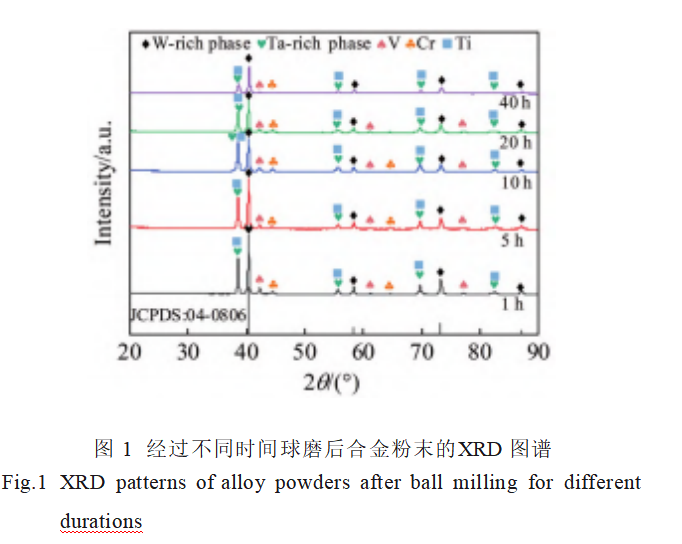
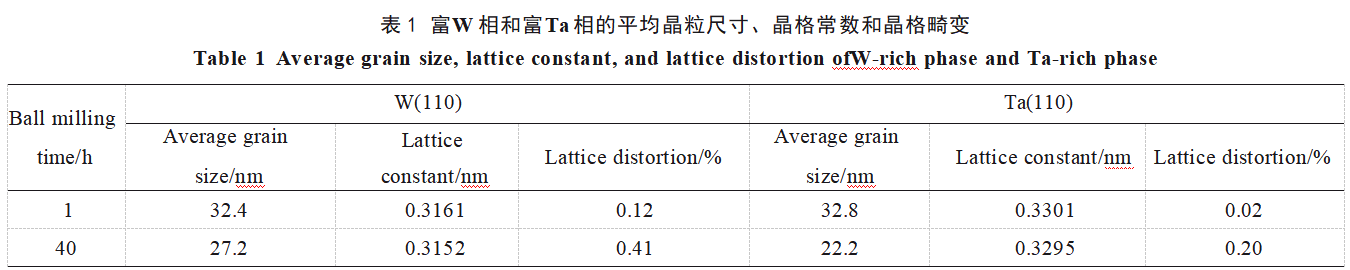
Furthermore, Table 1 indicates that compared to the alloy powder after 1 hour of ball milling, the average grain sizes of W and Ta after 40 hours of milling are smaller at 27.2 nm and 22.2 nm, respectively, with greater lattice distortions of 0.41% and 0.20%. This occurs because prolonged milling induces substantial plastic deformation, increasing strain in the grain regions and thereby elevating defect density. When the defect density in the grain boundary reaches a critical threshold, grain fragmentation occurs, leading to continuous grain refinement [25]. Furthermore, as ball milling time increases, more Cr and V dissolve into W and Ta. Due to the significant differences in atomic sizes among these elements, this induces greater lattice distortion.
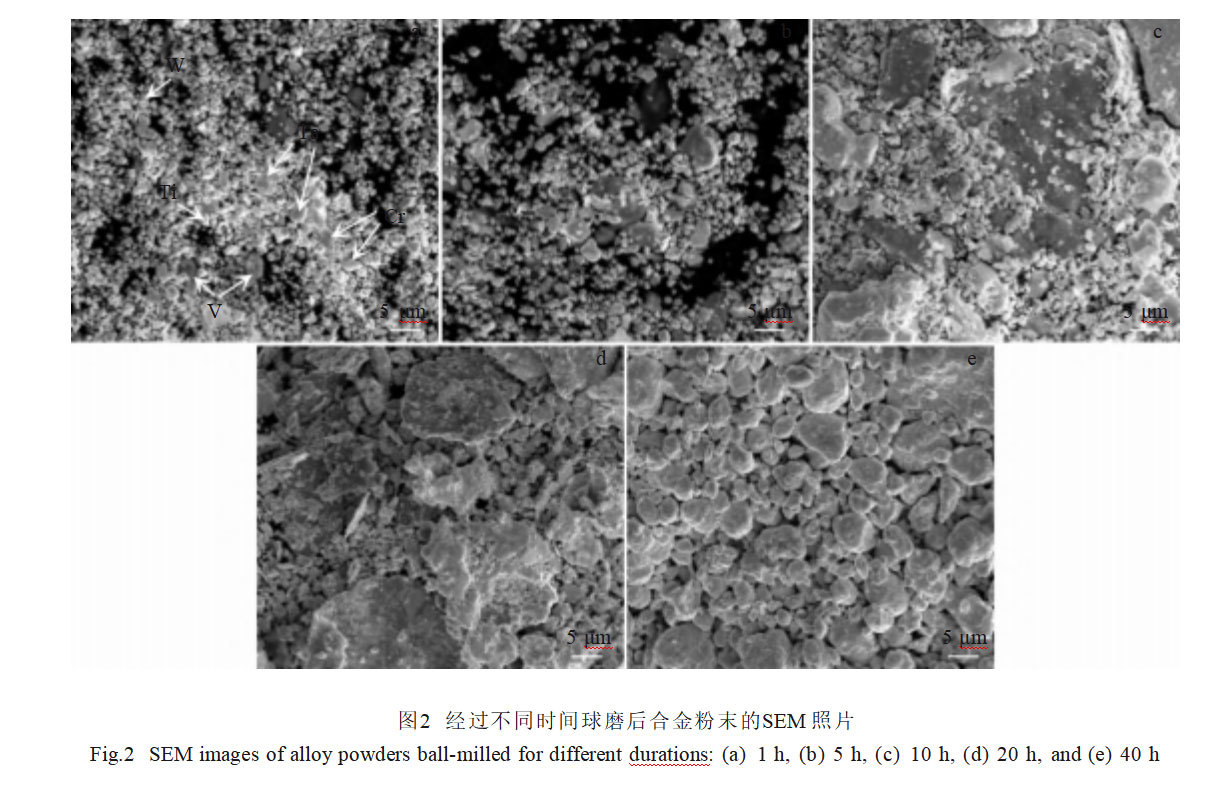
Figure 2 shows SEM images of the alloy powder after ball milling for different durations. The powder milled for 1 hour largely retains the original particle shapes of the constituent metals: W appears as regular polyhedral spherical particles (1.55±0.39 μm), Ta as spherical particles (3.53±0.61 μm), Cr as irregularly shaped particles, V as larger flake-like particles, and Ti as small spherical particles. After 5 hours of ball milling, the metal powders began to agglomerate, forming larger particles. Following 10 hours of milling, extensive agglomeration occurred, resulting in alloy powders predominantly composed of irregular, flake-shaped, and fragmentary particles of varying sizes. After 20 hours, the number of irregular flake-shaped particles increased further, reflecting a cyclical process of particle fragmentation into fine particles followed by cold welding of these fine particles. After 40 hours of ball milling, the alloy powder particle size tended toward uniformity at 3.65 ± 1.91 μm, exhibiting an equiaxed morphology.
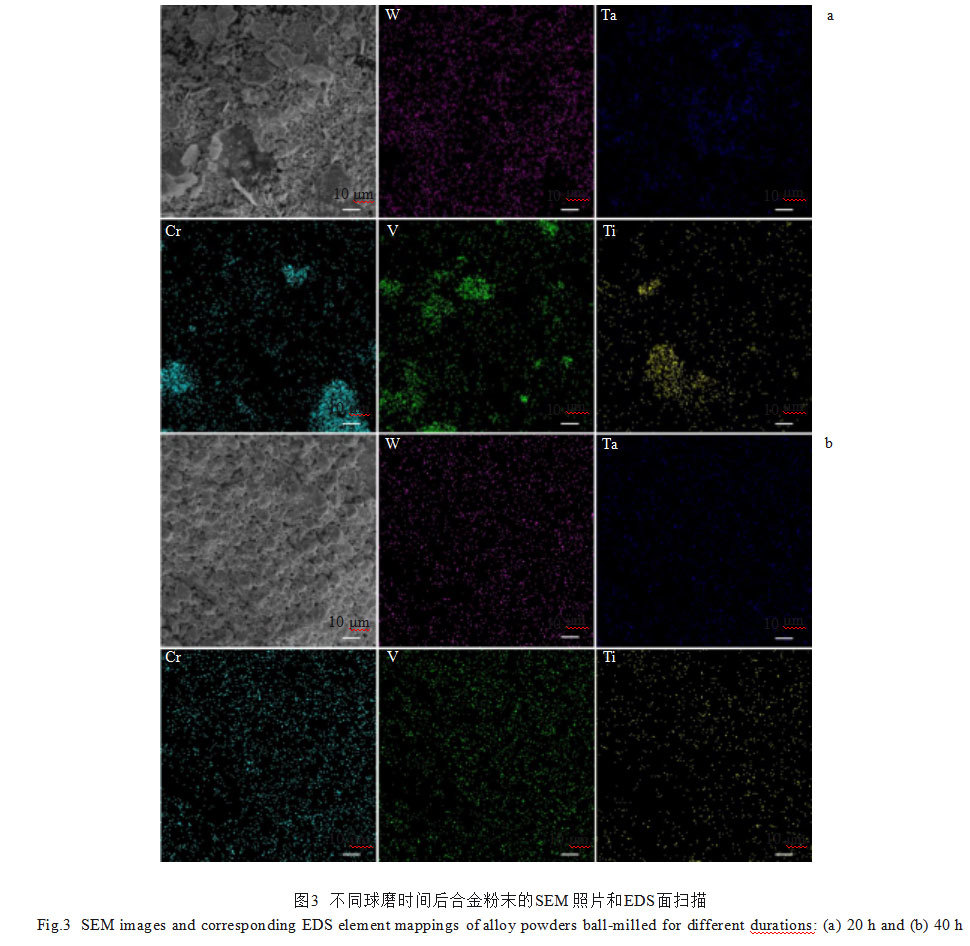
Figure 3 shows the EDS surface scan element distribution maps of the alloy powders after 20 and 40 hours of ball milling. In the 20-hour milled powder, W and Ta elements distributed relatively uniformly, while Cr, V, and Ti exhibited clustering. The 40-hour milled powder showed uniform distribution of all metallic elements with no elemental clustering observed.
3.2 Microstructure of Alloys After SPS Sintering
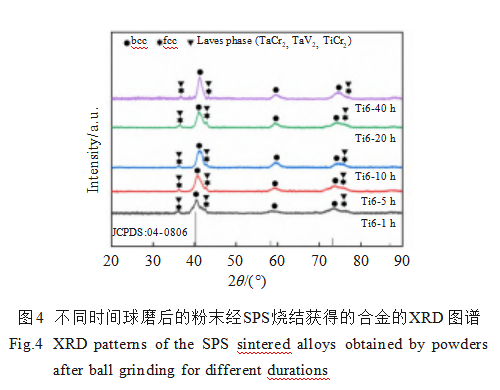
Alloy powders milled for different durations were sintered using SPS to produce alloy bulk specimens. Figure 4 shows the XRD patterns of each alloy. Diffraction peaks for the bcc phase, fcc phase, and Laves phase were observed in all alloy samples, whereas no Ta diffraction peak was detected in the original alloy powder. With increasing ball milling time, the intensity of the bcc phase diffraction peak gradually increased and the peak became sharper, indicating improved crystallinity. Conversely, the intensities of the fcc phase and Laves phase diffraction peaks gradually decreased. Particularly in the Ti6-40h alloy, the diffraction peaks of the fcc and Laves phases nearly disappeared. Additionally, its bcc phase diffraction peak shifted significantly toward higher angles, indicating extensive mutual atomic solid solution formation of a high-entropy alloy phase. To verify the formation of a high-entropy alloy in this system, relevant thermodynamic parameters were calculated according to Equations (6) to (11) [26-28], as follows:
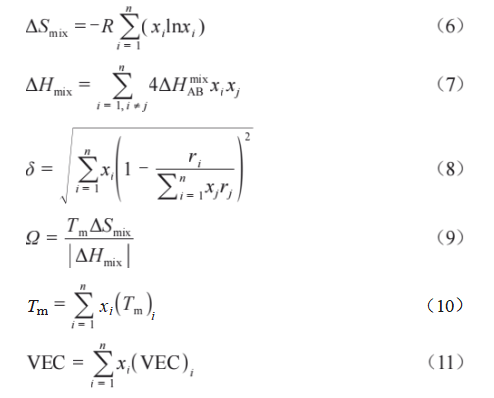
where R is the gas constant (8.31 J·mol⁻¹·K⁻¹), x_i is the atomic fraction of the i-th element in the alloy, ΔH_(ix) is the mixing enthalpy of the binary liquid phase alloy, ri is the atomic radius of component i in the alloy, Tm is the theoretical melting point of the n-component alloy system, (Tm)i is the melting point of the i-th component, and (VEC)i is the valence electron concentration of the i-th element.
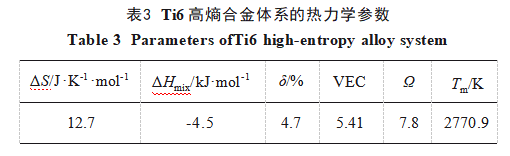
The calculation results are shown in Table 3. For a high-entropy alloy to form a solid solution, it must satisfy the following criteria [26-27]:
Ω > 1.1, δ < 6.6, -22 kJ/mol < ΔHmix < 7 kJ/mol, and 11 J/(K·mol) < ΔSmix < 19.5 J/(K·mol). Additionally, when the alloy's VEC < 6.87, the material tends to form a bcc structure. Here, Ω describes the entropy-enthalpy competition relationship, δ represents the atomic size difference parameter characterizing the combined effect, ΔHmix reflects chemical compatibility between elements, ΔSmix indicates system disorder, and VEC denotes valence electron concentration. Calculation results in Table 3 demonstrate that the WTaCrVTi6 alloy system satisfies the conditions for forming a high-entropy alloy with a bcc structure, a finding corroborated by XRD analysis.
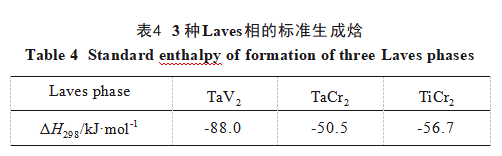
The formation of Laves phases is frequently observed in Cr-containing RHEAs [29-31]. This arises from the significant atomic radius difference between Cr and other elements, which favors the formation of AB₂ type Laves phases such as TaCr₂. Additionally, the addition of Ti may lead to the formation of other Laves phases in the alloy, including TaV₂, VTa₂, and TiCr₂ [20]. For high-entropy alloys, intermetallic compounds or intermediate phases form when their standard enthalpy of formation exceeds the high-entropy effect. Table 4 lists the standard enthalpies of formation for three potential Laves phases in this alloy [32], all lower than that of Ti6. Thus, the Laves phases in this alloy may include TaCr₂, TaV₂, and TiCr₂.
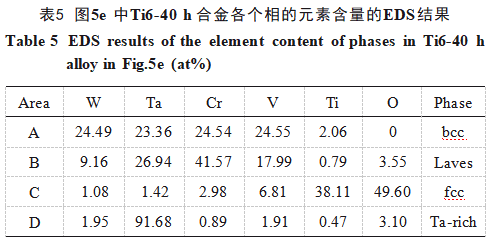
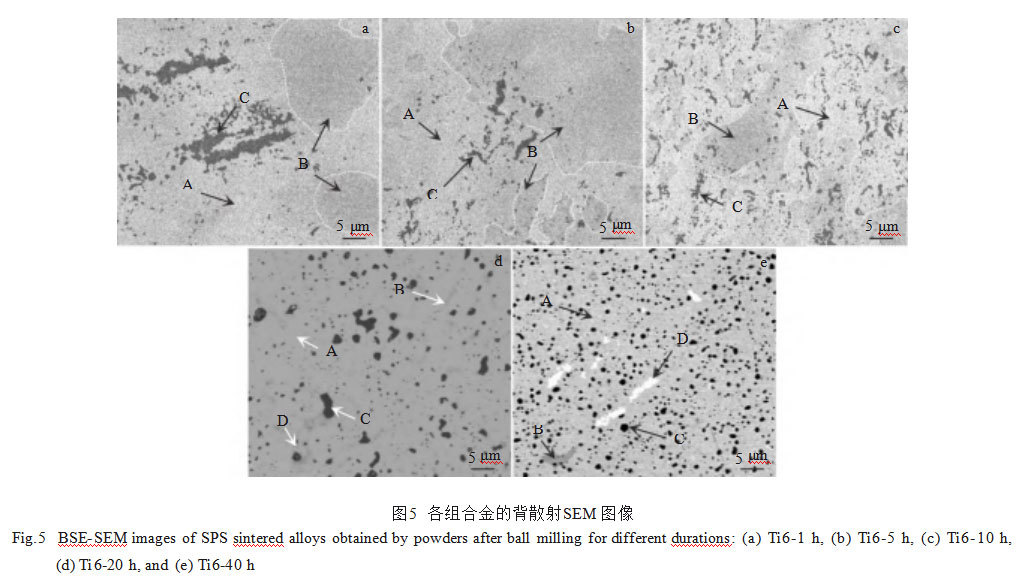
Figure 5 shows backscattered SEM images of the surfaces of each alloy. As seen, all alloys exhibit dense microstructures with no obvious voids. The densities of each alloy were measured using the displacement method, with relative densities exceeding 99.0%. All alloys contain grayish-white Phase A, grayish-black Phase B, and black Phase C. Notably, white Phase D also appears in the Ti6-20h and Ti6-40h alloys. EDS analysis was performed on each sample. Table 5 presents the EDS results for the Ti6-40h alloy, while Figure 6 shows its EPMA image. Integrating these with the aforementioned XRD results reveals: the gray-white Phase A exhibits near-equimolar ratios of W, Ta, Cr, and V, corresponding to a bcc WTaCrVTix solid solution; the gray-black Phase B is enriched in Ta, Cr, and V, representing a Laves phase; The black phase C exhibits high Ti and O content with an atomic ratio close to 1:1, and TiO adopts an fcc structure, confirming phase C as TiO. The white phase D is a Ta-enriched phase. Figures 5a–5c reveal that the microstructures of alloys prepared from powders subjected to short ball milling times (1, 5, and 10 h) exhibit non-uniformity. Black TiO₂ particles aggregate, while extensive Laves phases are irregularly distributed throughout the matrix. As ball milling time increased, the black TiO₂ particles began to disperse, the area of the Laves phase decreased significantly, and Ta-rich phases appeared around it. The microstructure of the alloy started to become uniform, as shown in Figure 5d. In the Ti6-40h alloy, as shown in Figure 5e, the bcc phase was continuously distributed, and the black TiO₂ particles were uniformly distributed in the matrix with an average particle size of 1.08 ± 0.38 μm. The Laves phase nearly disappeared, replaced by Ta-enriched phases exhibiting similar morphology, size, and distribution to the Laves phase. This occurs because prolonged ball milling reduces the average grain size of the alloy powder and promotes the dispersion of elements. This facilitates diffusion of Cr and V from the Laves phase into the bcc phase during sintering, depleting Cr and V in the original Laves phase and resulting in the formation of white Ta-enriched phases.
To investigate the effect of ball milling time on the composition of the primary bcc phase in high-entropy alloys, Table 6 presents the elemental contents within the bcc phase for alloys prepared with varying milling durations. The table indicates that during shorter ball milling times (1–10 h), the Cr content in the bcc phase is relatively low. As milling time increases, particularly in the alloy milled for 40 h, the atomic ratios of W, Ta, Cr, and V in the bcc phase approach stoichiometry. This indicates that extended ball milling promotes diffusion among W, Ta, Cr, and V atoms—all sharing the bcc structure—facilitating the formation of bcc solid solutions during sintering. Meanwhile, Ti, which adopts an hcp structure at room temperature, undergoes allotropic transformation to bcc at elevated temperatures. However, Ti exhibits limited solid solubility in W, resulting in lower Ti content within the bcc phase. Furthermore, Ti possesses strong oxygen affinity; during sintering, it reacts with residual oxygen in the powder to form black TiO₂.
3.3 Mechanical Properties
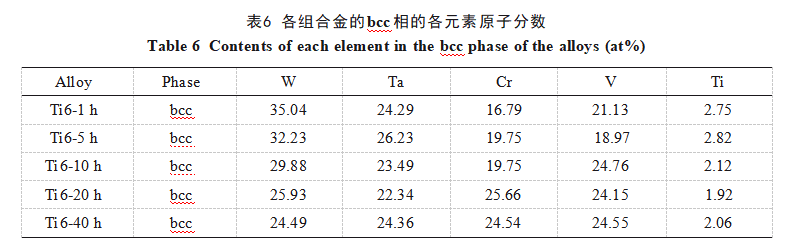
Figure 7 shows the room-temperature and high-temperature compression curves of the Ti6-40h alloy. The compression yield strengths at room temperature and high temperature are 2870 and 1954 MPa, respectively, with a room-temperature microhardness of 873.4 ± 7.6 HV. Table 7 lists the relevant mechanical property data for the two alloys prepared using MA and SPS techniques, compared with WTaCrVTi alloys prepared by other researchers. As shown in the table, the WTaCrVTi6 alloy prepared in this study exhibits significantly superior mechanical properties compared to RHEAs produced by other methods, owing to its uniform microstructure.
The strengthening mechanisms of high-entropy alloys include solution strengthening, second-phase strengthening, and grain refinement, with solution strengthening being the primary mechanism. First, the bcc-structured elements W, Ta, Cr, and V form a bcc solid solution. However, the substantial differences in atomic radii among these four atoms hinder the formation of a homogeneous solid solution. Therefore, preparing the Ti6 alloy via the MA combined with SPS method and adding an appropriate amount of Ti[20] promotes the formation of an equimolar solid solution, maximizing its mixed entropy and solid solution strengthening effect. Second, the complex mixed entropy and mixed enthalpy relationships among components in the WTaCrVTi alloy system readily form Laves phases and TiO phases. These phases induce pinning effects, stabilizing the grain structure, impeding dislocation and crack propagation, and contributing to secondary phase strengthening. Finally, the addition of an appropriate amount of Ti promotes grain refinement in the alloy, achieving grain refinement strengthening. The Ti6-40h alloy exhibits a uniform microstructure, resulting in excellent compressive strength and microhardness. At elevated temperatures, the compressive strength of Ti6-40h alloy decreases significantly. This may be attributed to softening of the low-melting-point TiO₂ phase, reducing the alloy's high-temperature compressive strength. Nevertheless, the alloy retains a high-temperature compressive strength of 1954 MPa, demonstrating excellent resistance to softening at elevated temperatures.
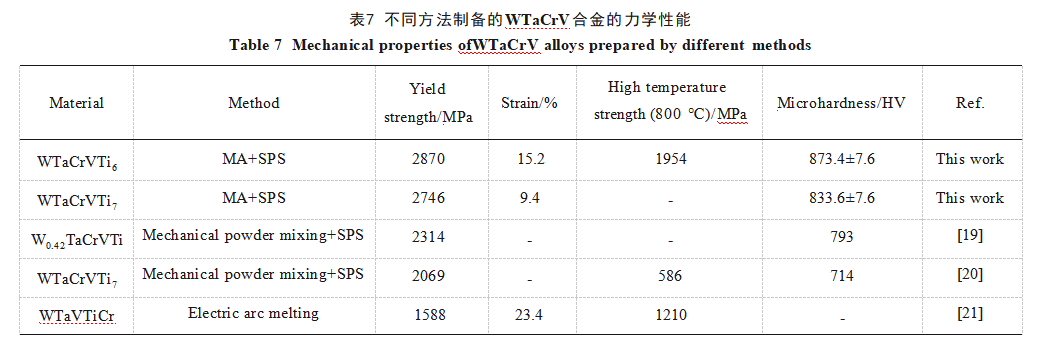
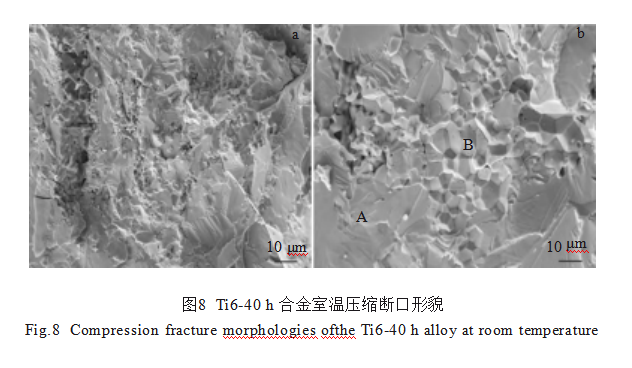
Figure 8 shows the fracture morphology of the Ti6-40h alloy after compression. The fracture surface exhibits both river-like intergranular cleavage planes (marked as A) and ice-cube-like intergranular fracture features (marked as B). This indicates a mixed fracture mode of intergranular and transgranular fracture.
4 Conclusions
1) With extended ball milling time, the alloy powder composition tends toward homogenization, grain size decreases, and lattice distortion increases. Powder milled for 40 hours exhibits uniform particle size of approximately 3.65 ± 1.91 μm with an equiaxed morphology. The average grain sizes of W and Ta are 27.2 nm and 22.2 nm, respectively, with lattice distortions of 0.41% and 0.20%. This is attributed to the solid solution of Cr and V—atoms with smaller atomic radii—into W and Ta.
2) SPS sintering of powders milled for different durations yielded dense alloys with relative densities exceeding 99.0%. The alloys contained bcc, fcc, and Laves phases: the bcc phase was a WTaCrVTix solid solution, the fcc phase was TiO, and the Laves phases included TaCr₂, TaV₂, and TiCr₂, among others. The Ti6-40h alloy exhibits uniform microstructure: continuous distribution of the bcc phase with near-equimolar ratios of W, Ta, Cr, and V atoms; significantly reduced Laves phases; and uniformly distributed TiO particles in the matrix phase with an average size of 1.08±0.38 μm.
3) The Ti6-40h alloy exhibits outstanding room-temperature/high-temperature compressive yield strength and microhardness, measuring 2870 and 1954 MPa, respectively, with a microhardness of 873.4±7.6 HV. Extended ball milling promotes the formation of an equimolar solid solution, maximizing mixed entropy and solid solution strengthening effects. The fracture mode of the Ti6-40h alloy is a mixture of intergranular and transgranular fracture.
References: Chinese Library Classification: TG139; TG132.3+3 Document Code: A Article Number: 1002-185X(2025)09-2368-09 Microstructure and Mechanical Properties of Refractory High-Entropy Alloys WTaCrVTi Prepared by MA-SPS Method
Stardust Technology employs radiofrequency plasma spheroidization technology to produce high-performance spherical refractory high-entropy alloy powders, primarily encompassing W-Mo-Ta-Nb, TaNbVTi, and other systems. These powders feature high sphericity, high purity, low oxygen content, excellent flowability, and high density, making them suitable for aerospace (engine components, rocket nozzles), nuclear industry, military equipment, and additive manufacturing (3D printing) applications. The company offers customized particle size specifications (e.g., 15-53μm, 45-105μm) and composition designs to meet extreme operational demands such as high temperatures, corrosion resistance, and high strength. For further custom refractory high-entropy alloy powder services, please contact our professional manager Cathie Zheng at +86 13318326187.

News

