Determination of Tantalum and Niobium in Tungsten Slag Using Inductively Coupled Plasma Atomic Emission Spectroscopy
Release time:
2025-08-14
0 Introduction
In 2016, alkali leaching residue (tungsten slag) produced by the alkali decomposition method was officially included in the National List of Hazardous Wastes [1]. Over the past few decades, China has accumulated millions of tons of tungsten slag, with an annual increase of nearly 80,000 tons. A large amount of tungsten slag urgently requires harmless treatment and resource utilization [2-3]. Tantalum and niobium are important associated elements in tungsten concentrate. They are insoluble in conventional concentrations of sodium hydroxide (NaOH) and potassium hydroxide (KOH) solutions. Therefore, tantalum and niobium from tungsten ore enter the tungsten slag during tungsten smelting, with (Ta₂O₅ + Nb₂O₅) content in the slag reaching 0.2%–7.94% [4–9]. Tantalum and niobium possess high melting points, good plasticity, excellent electrical and thermal conductivity, and strong corrosion resistance, making them widely used in military, electronics, aerospace, and superconducting technology industries. With the rapid development of modern industry, the demand for tantalum and niobium has surged, while China's reserves of these resources are extremely limited, and extraction costs are high. Therefore, the recovery and utilization of tantalum and niobium resources from tungsten slag are of great significance, and accurately determining the content of tantalum and niobium in tungsten slag can provide a reference for the recovery and utilization of these resources.
Currently, the main analytical methods for determining tantalum and niobium include paper chromatography-gravimetric method [11], spectrophotometric method [12], inductively coupled plasma emission spectroscopy [12-13], and inductively coupled plasma mass spectrometry [13]. The paper chromatography-gravimetric method is suitable for determining high concentrations of tantalum and niobium in tantalum-niobium concentrates, while the inductively coupled plasma mass spectrometry method is suitable for determining trace amounts of tantalum and niobium. The tantalum and niobium content in tungsten slag falls between trace and macro levels, making neither of the above two methods applicable. Although spectrophotometry can meet the requirements for determining tantalum and niobium content in tungsten slag, it has a lengthy detection process and is subject to numerous interference factors. In summary, this study employs inductively coupled plasma emission spectroscopy to determine the tantalum and niobium content in tungsten slag, and examines key factors such as sample decomposition methods, selection of analytical spectral lines, interference from coexisting ions, and reagent quantities, with the aim of developing a detection method that is simple to operate, fast in analysis, minimally affected by interference, has a wide linear range, and high sensitivity, to meet the requirements for determining the tantalum and niobium content in tungsten slag.
1 Experimental Section
1.1 Instruments and Reagents
Ultima2 Inductively Coupled Plasma Atomic Emission Spectrometer (Horiba, Japan), equipped with a hydrogen fluoride-resistant sample introduction system. Sodium peroxide (analytical grade); hydrochloric acid (1.19 g/mL); hydrogen fluoride (1.15 g/mL); Tantalum and niobium standard storage solutions: Transfer 10.00 mL of tantalum (GSB04-1755—2004) and niobium (GSB04-1740—2004) certified standard solutions into 100 mL plastic volumetric flasks, dilute to the mark with hydrofluoric acid solution (1+19), and mix thoroughly. This solution contains 100 μg of tantalum and niobium per 1 mL.
1.2 Method Principle
Tantalum and niobium in tungsten slag exist in the form of FeTa₂O₆ and FeNb₂O₆. When melted with sodium peroxide, they form corresponding tantalum and niobium salts. Since sodium niobate and sodium tantalate are insoluble in water, adding hydrochloric acid and hydrofluoric acid forms the fluorides TaF₅ and NbF₅, which enter the solution. Argon plasma spectroscopy is then used for determination. A blank solution is used to prepare the standard solution to eliminate the influence of sodium and other matrix ions on the determination. 1.2 Analytical Method Weigh 0.5000 g of tungsten slag sample into an iron crucible (previously loaded with 1 g of sodium peroxide), add 3 g of sodium peroxide, mix thoroughly with a plastic rod, then uniformly cover the surface with 1 g of sodium peroxide. Place in a 750°C muffle furnace and melt for approximately 5 minutes until cherry red, then remove and allow to cool slightly. Place the crucible in a 400 mL plastic beaker (previously filled with 100 mL of water). After vigorous reaction ceases, add 5 mL of hydrofluoric acid and stir thoroughly. Under constant stirring, add hydrochloric acid dropwise to acidify the solution. After the solution becomes clear, add 2 mL of hydrochloric acid, cool to room temperature, transfer to a 250 mL plastic volumetric flask, dilute with water to the mark, mix thoroughly, and filter. Transfer 5.00 mL of the test solution to a 50 mL plastic volumetric flask, dilute with water to the mark, and mix thoroughly. Determine the analytical test solution and standard series solutions simultaneously.
1.3 Preparation of Working Standard Solutions
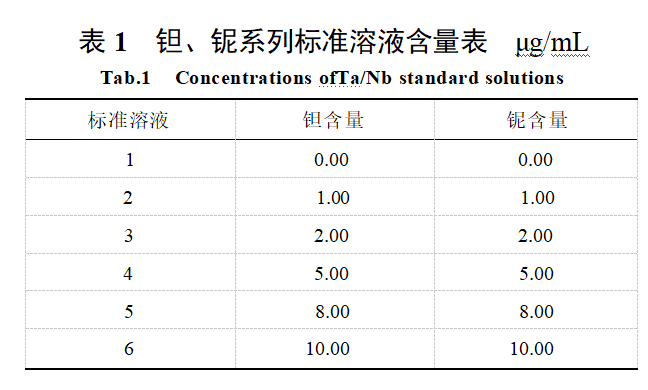
Prepare blank solutions according to the analytical method. Transfer 6 portions of 10.00 mL blank solution into 6 100 mL volumetric flasks. Transfer 0 mL, 1.00 mL, 2.00 mL, 5.00 mL, 8.00 mL, and 10.00 mL of tantalum-niobium standard storage solution into 6 100 mL volumetric flasks, Add 2 mL of hydrochloric acid and 2 mL of hydrofluoric acid to each, dilute with water to the mark, and mix thoroughly. The tantalum and niobium content in each standard solution is shown in Table 1.
2 Results and Discussion
2.1 Selection of Sample Digestion Methods
The main methods for digesting tantalum and niobium include alkali fusion, microwave digestion, and high-pressure reactor digestion. These three methods were used to digest the 1# tungsten slag sample.
Microwave digestion method: Place the sample in a digestion vessel, add 3–5 mL of water to moisten it, then add 2 mL of hydrofluoric acid and 1 mL of nitric acid in sequence, ensuring thorough mixing of the sample and digestion solution. If a vigorous chemical reaction occurs, wait until the reaction has ceased before sealing the vessel tightly. Transfer the vessel to a microwave digestion system, heat to 180°C and maintain for 10 minutes, then continue heating to 220°C and maintain for 20 minutes before shutting off the system. After cooling, filter the digestion solution from the vessel using slow-speed filter paper, wash the precipitate with 2% HF three to four times, and perform alkaline fusion of the filter paper and precipitate. The resulting residue is then analyzed using inductively coupled plasma mass spectrometry (ICP-MS).
High-pressure reactor digestion method: Place the sample in a high-pressure reactor, add 3–5 mL of water to moisten it, then add 2 mL of hydrofluoric acid and 1 mL of nitric acid in sequence to ensure thorough mixing of the sample and acid solution. If a vigorous chemical reaction occurs, wait until the reaction has ceased before closing and tightening the reactor lid. Place the reactor in a drying oven, heat to 180°C, react for 4 hours, remove, and wait until the reactor temperature has cooled to room temperature before opening. Filter the reaction solution in the reactor using slow-speed filter paper, wash the precipitate with 2% HF three to four times, and perform alkaline fusion on the filter paper and precipitate. The resulting residue is then analyzed using inductively coupled plasma mass spectrometry (ICP-MS).
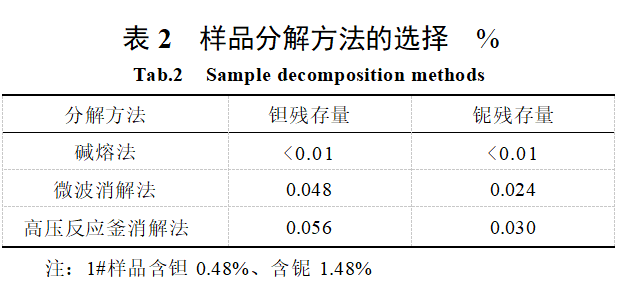
The results of the decomposition methods for different samples are shown in Table 2. As shown in Table 2, the alkaline fusion method yields the lowest tantalum and niobium residue, meeting the detection requirements. When melting tungsten slag with sodium hydroxide or sodium carbonate, the resulting tantalum sodium and niobium sodium are insoluble in high-salt solutions. However, when using sodium peroxide for melting, the resulting per-tantalum salts and per-niobium salts are soluble in water [14]. Therefore, this experiment employs the sodium peroxide alkali fusion method to decompose tantalum and niobium from tungsten slag.
2.2 Selection of Hydrofluoric Acid Dosage
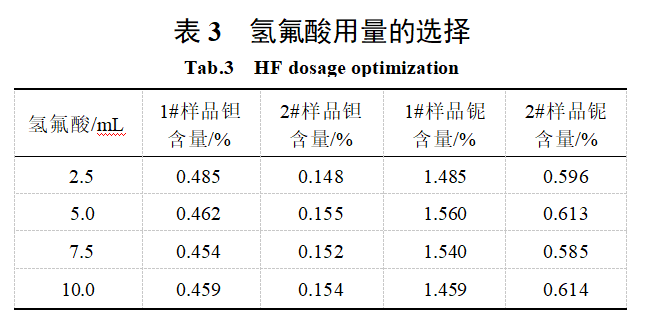
After the sample was leached by alkaline fusion, hydrofluoric acid was added to form a complex, and then the solution was acidified with hydrochloric acid, allowing tantalum and niobium to enter the solution. Hydrofluoric acid was added in amounts of 2.5 mL, 5.0 mL, 7.5 mL, and 10.0 mL to determine the tantalum and niobium content in samples 1# and 2# of tungsten slag. The results are shown in Table 3. The results indicate that hydrofluoric acid dosages ranging from 2.50 to 10.0 mL can effectively complex tungsten and other elements, with consistent measurement results. Therefore, a hydrofluoric acid dosage of 5 mL was selected.
2.3 Selection of Measurement Method
After decomposing the sample using the alkali fusion method, it was diluted to a final volume of 250 mL in a volumetric flask. The sodium concentration was approximately 25 mg/mL, requiring further dilution before determination. A blank standard was used to establish the calibration curve. To assess sodium interference, 100 μg each of tantalum and niobium were added to the diluted blank solution, and the recovery rate was measured. The results are shown in Table 4.
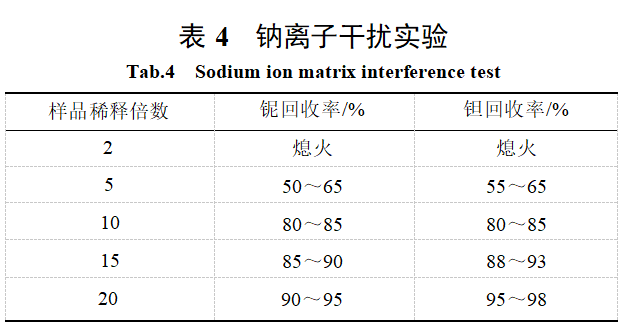
As shown in Table 4, the influence of sodium ions on the measurement gradually decreases with increasing dilution factor, and the change becomes smaller after a 10-fold dilution. Considering factors such as the detection limit, this experiment selected a dilution factor of 10-fold, matched the standard solution with the blank solution, and adopted the approximate matrix matching method for measurement.
2.4 Coexisting Element Interference and Selection of Analytical Spectral Lines
The chemical element composition of tungsten slag varies due to differences in the raw material composition of tungsten minerals, primarily including tungsten, iron, manganese, calcium, silicon, fluorine, and arsenic, as well as additives from the smelting process [3]. An experimental mixture of coexisting elements was simulated, with the following concentrations: Nb (2 μg/mL), Ta (2 μg/mL), Si (10 μg/mL), Ca (20 μg/mL), Fe (20 μg/mL), Mn (20 μg/mL), W (20 μg/mL). The tantalum and niobium content was determined at different tantalum-niobium spectral lines, and the recovery rate was calculated. The recovery rates obtained from the tantalum 263.558 nm and niobium 309.418 nm spectral lines were closest to 100%, so the analytical spectral lines were selected as tantalum 263.558 nm and niobium
309.418 nm.
2.5 Detection Limit, Quantification Limit, and Determination Range
Coexisting elements were added to the blank solution, with concentrations of Si solution (10 μg/mL), Ca (20 μg/mL), Fe (20 μg/mL), Mn (20 μg/mL), W (20 μg/mL). The blank solution was subjected to 11 parallel determinations, with a standard deviation of 0.0015 μg/mL. The detection limit was calculated as 0.0045 μg/mL based on 3σ. The detection limit was determined to be 0.01% based on the sample weight [13], dilution factor, and 10σ, with a determination value of 0.0075%. . Multiple working curves were constructed using the standard series, and the linear correlation coefficients for tantalum and niobium were all greater than 0.9995, indicating that the working curve was linear and met the detection requirements. Based on the standard curve and the lower limit of detection, the detection range for tantalum and niobium was determined to be 0.01% to 4.00%.
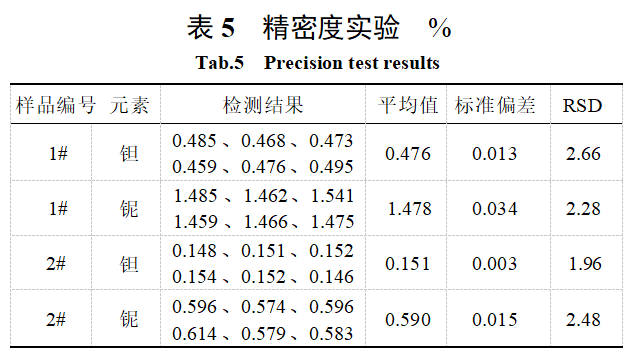
2.6 Precision Experiment
The precision of the method was evaluated by conducting six parallel determination experiments on samples 1# and 2# tungsten slag. Experimental samples: Sample 1# was derived from production raw materials primarily consisting of black tungsten concentrate, while sample 2# was derived from production raw materials primarily consisting of mixed tungsten concentrate. The determination results are shown in Table 5. As shown in Table 5, the relative standard deviation (RSD) of the samples is less than 3%, indicating that the method meets the analytical requirements.
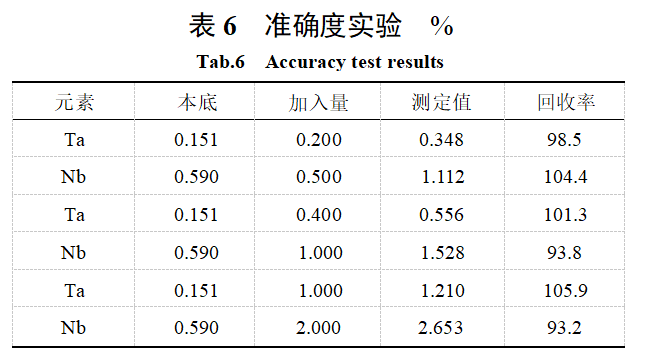
2.7 Accuracy Experiment
The accuracy of the method was verified using the standard addition method. The standard addition amounts and detection results are shown in Table 6. As shown in Table 6, the recovery rate of tantalum and niobium ranges from 96% to 104%, indicating that the accuracy of the method meets the analytical testing requirements.
3 Conclusions
This study established a method for determining the content of tantalum and niobium in tungsten slag samples by alkaline fusion decomposition of sodium peroxide, complexing tungsten and other impurity ions with hydrofluoric acid, and measuring the content of tantalum and niobium in the tungsten slag using argon plasma emission spectroscopy under acidic conditions. The method has a measurement range of 0.01% to 4%, with relative standard deviations (RSD) all below 3%, and recovery rates for tantalum and niobium ranging from 96% to 104%. It features simple operation, good precision, a wide measurement range, and high accuracy, making it suitable for routine analysis of tantalum and niobium in tungsten slag.
References: Chinese Library Classification: O657.31 Document Identification Code: A
Determination of tantalum and niobium in tungsten slag using inductively coupled plasma atomic emission spectroscopy
Xingchen Technology (Guangdong) Co., Ltd. specializes in the research, development, and production of high-performance spherical metal powders. Spherical tungsten powder and spherical niobium powder are produced using advanced radio frequency plasma spheroidization technology, featuring high purity (>99.95%), low oxygen content, excellent sphericity, and flowability, making them suitable for 3D printing, additive manufacturing, and high-temperature applications. Spherical tungsten powder demonstrates exceptional high-temperature strength and corrosion resistance in aerospace and nuclear industries, while spherical niobium powder (such as C103 niobium-hafnium alloy) offers low density, high-temperature strength, and excellent processability, making it suitable for engine components and medical device manufacturing. Both powders are available with customized particle size distributions to meet various process requirements, such as Selective Laser Melting (SLM) and Electron Beam Melting (EBM). Please contact our professional manager Cathie Zheng, +86 13318326187.


