The Relationship Between Molybdenum Powder Particle Size and Specific Surface Area
Release time:
2025-08-06
Molybdenum and molybdenum alloys possess advantages such as high melting point, excellent electrical and thermal conductivity, corrosion resistance, low thermal expansion coefficient, and good thermal shock resistance, making them widely used in fields such as electronics, machinery, metallurgy, nuclear power [1], and aerospace [2]. Molybdenum powder is the raw material for producing molybdenum products, and its quality ensures the superior performance of molybdenum and molybdenum alloys [3-4]. In addition to meeting requirements for high purity of the main component and low impurity content, molybdenum powder must also satisfy relevant physical property requirements. Factors such as particle size, microstructure, particle size distribution, and specific surface area significantly influence the microstructure and properties of molybdenum products.
Particle size is one of the important physical properties of molybdenum powder. Generally, the smaller the particle size of molybdenum powder, the larger its surface area and the higher its reactivity. The particle size and particle size distribution of molybdenum powder directly affect its quality and application performance. Specific surface area refers to the total area per unit mass of material, with the unit being m²/g. Specific surface area is one of the key parameters for evaluating the physical properties of molybdenum powder. It is related to the size and morphology of molybdenum powder particles. Generally, the larger the specific surface area, the higher the reactivity and better the performance of molybdenum powder in reactions. Therefore, it is essential to control the specific surface area of molybdenum powder during production to ensure its quality.
Industry technicians have conducted extensive research on the physical properties of molybdenum powder. Xing Yanning et al. [5] studied the effects of factors such as particle size distribution, Fehrer particle size, bulk density, tapped density, and powder morphology on the flexural strength of molybdenum powder briquettes. They found that the flexural strength of molybdenum powder briquettes primarily depends on the agglomeration status and particle size distribution of the molybdenum powder particles. the fewer agglomerates in the molybdenum powder and the closer the particle size distribution is to a standard normal distribution, the greater the flexural strength. Chen Yanfang et al. [6] prepared pure molybdenum plates using molybdenum powder with different physical properties and found that pure molybdenum plates prepared using molybdenum
powder with low agglomeration, good dispersion, and relatively uniform particle size had a uniform and dense structure, higher hardness, and better tensile properties. Therefore, the physical properties of molybdenum powder can be improved through processing. Zhang Changle et al. [7] and Wu Zhou et al. [8] respectively studied the effects of mechanical processing and airflow milling on the physical properties of molybdenum powder. After processing, the Fritsch particle size, bulk density, tapped particle size, and particle size distribution of the molybdenum powder were all improved. The physical properties of molybdenum powder influence the microstructural properties of molybdenum products, particularly the particle size and specific surface area of the molybdenum powder. Currently, there is limited research on the relationship between molybdenum powder particle size and specific surface area. The author investigated the relationship between molybdenum powder particle size and specific surface area, exploring the relationship between the Fritsch particle size, particle size distribution, and microscopic particle size of molybdenum powder and its specific surface area. The results can provide guiding assistance for the production and testing of molybdenum powder.
1 Experimental Methods
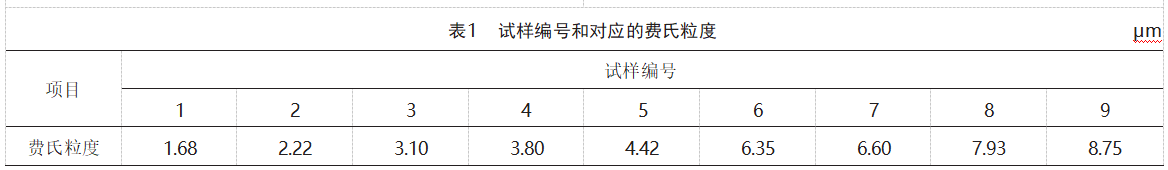
Nine groups of molybdenum powder with increasing Fehrer particle sizes were selected for testing. The sample numbers and corresponding Fehrer particle sizes are shown in Table 1.
The Fehrer particle size of the molybdenum powder was determined using an average particle size analyzer, and the specific surface area was measured using a specific surface area pore size analyzer with nitrogen adsorption. The microstructure of the molybdenum powder was observed using a scanning electron microscope (SEM) [9], and five random positions were selected, with at least 15 molybdenum powder particles measured at each position to calculate the average particle size, which was used as the micro-particle size of the molybdenum powder. The particle size distribution of molybdenum powder was determined using a laser diffraction particle size analyzer. To reduce the influence of molybdenum powder agglomeration on the particle size distribution test results, ethylene glycol was used as a dispersant to ultrasonically disperse the molybdenum powder, followed by particle size distribution testing.
2 Theoretical Calculation
Assuming that all molybdenum powder particles are agglomerate-free, uniform in size, and dense spherical particles, the relationship between the specific surface area (B) and the particle size (d) of the molybdenum powder is shown in Equation (1).
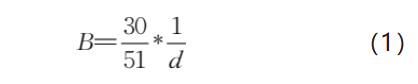
From Equation (1), it can be seen that the specific surface area of molybdenum powder is inversely proportional to its particle size; the larger the particle size, the smaller the specific surface area.
3 Experimental Results
The measurement results of the Mo powder's Fritsch particle size, microscopic particle size, particle size distribution, and specific surface area are shown in Table 2. Due to the presence of agglomerates in the molybdenum powder, agglomerates can affect the measurement results during particle size distribution testing [10], failing to reflect the true particle size of the sample. Therefore, the particle size distribution results of the sample are higher than the Fehrert particle size. However, in the particle size distribution results, D10 (the particle size corresponding to a cumulative particle size distribution percentage of 10%), D50 (the particle size corresponding to a cumulative particle size distribution percentage of 50%), D90 (particle size corresponding to a cumulative particle size distribution percentage of 90%) generally increase with the increase in the Fritsch particle size. Both the Fritsch particle size and the microscopic particle size are methods for characterizing the average particle size of molybdenum powder. The microscopic particle size measured using SEM is closest to the actual average particle size of molybdenum powder, and its statistical results are slightly lower than the Fritsch particle size results. Samples 8 and 9 have severe agglomeration, resulting in abnormally high Fritsch particle size results.
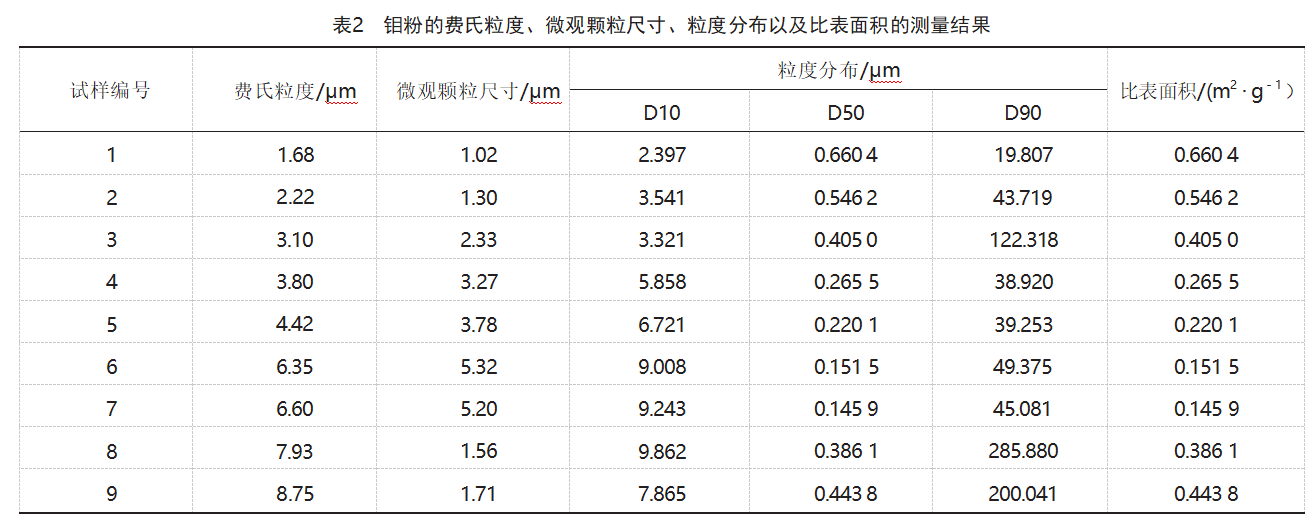
3.1 Microstructure
Figure 1 shows the SEM morphology of the molybdenum powder samples. As shown in Figure 1: Sample 1 molybdenum powder particles are spherical in shape, with relatively uniform particle sizes, but some agglomeration is present; Samples 2 and 3 molybdenum powder particles exhibit a coral-like morphology, with some larger agglomerated particles present; Samples 4–7 molybdenum powder particles are spherical in shape, with no obvious agglomeration observed; Specimens 8 and 9 exhibit severe agglomeration, with small molybdenum powder particles having combined to form hard agglomerates with sizes ranging from 50 to 250 μm.
3.2 Particle Size Distribution
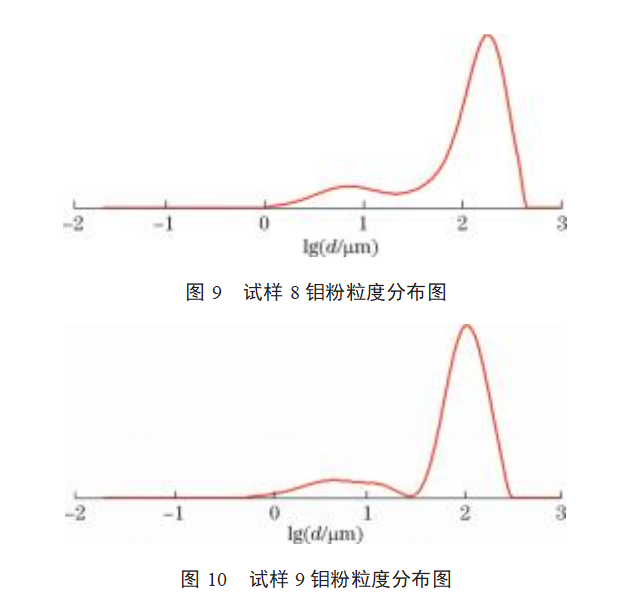
The particle size distributions of samples 1–9 are shown in Figures 2–10. As shown in Figures 2–10: The particle size distribution curves of samples 4–7 are single-peaked, and the particle size distribution generally follows a normal distribution; Sample 1 exhibits some agglomeration, resulting in a tailing trend in the particle size distribution curve; Samples 2 and 3 exhibit coral-like particle morphology with severe agglomeration, resulting in a bimodal particle size distribution curve; samples 8 and 9 exhibit severe particle agglomeration, leading to a more pronounced bimodal distribution curve with a pronounced two-tier differentiation between small and large particle sizes. This indicates that the more severe the agglomeration of molybdenum powder, the more pronounced the double-peak phenomenon in the particle size distribution curve. Molybdenum powder with less severe agglomeration exhibits a single peak in the particle size distribution curve, following a normal distribution.
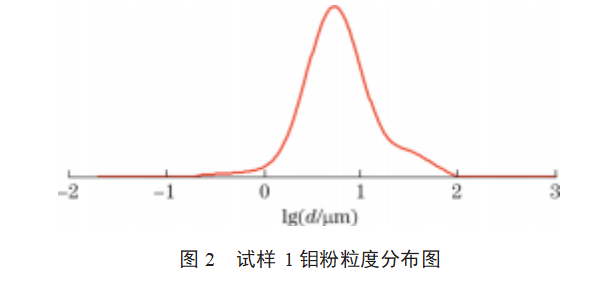
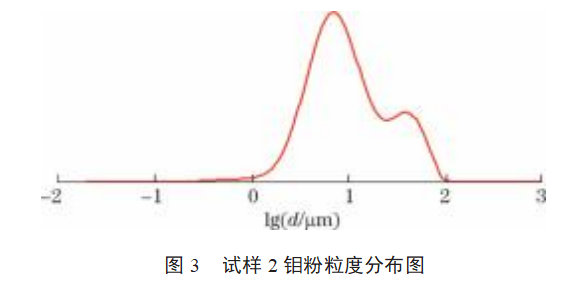
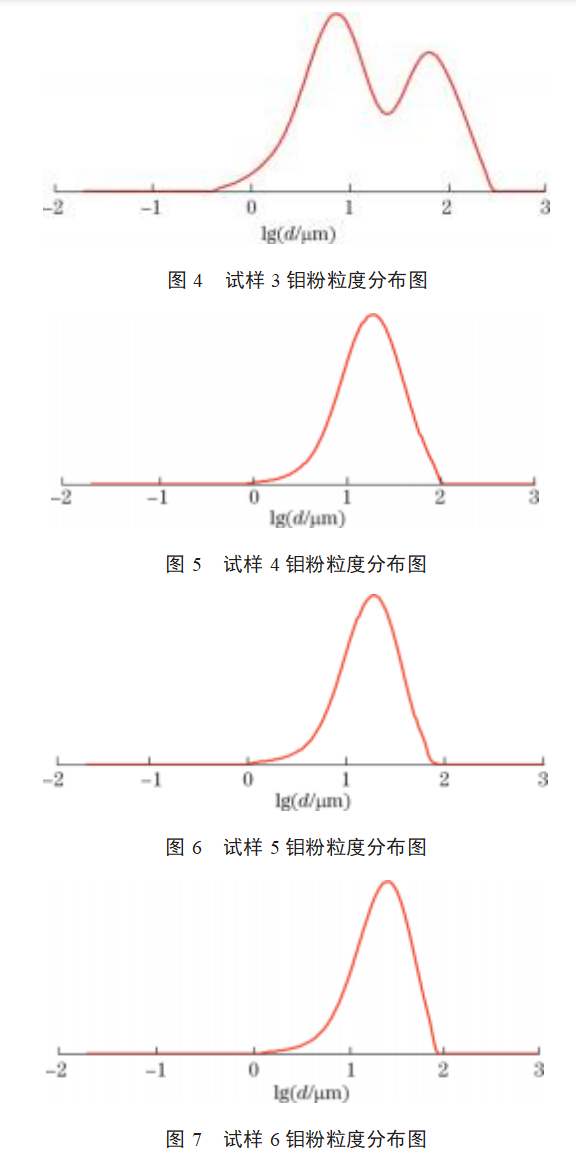
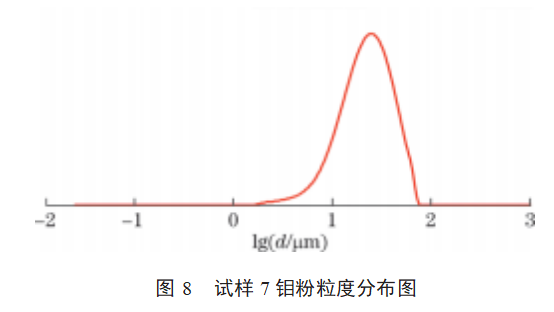
3.3 Data Analysis
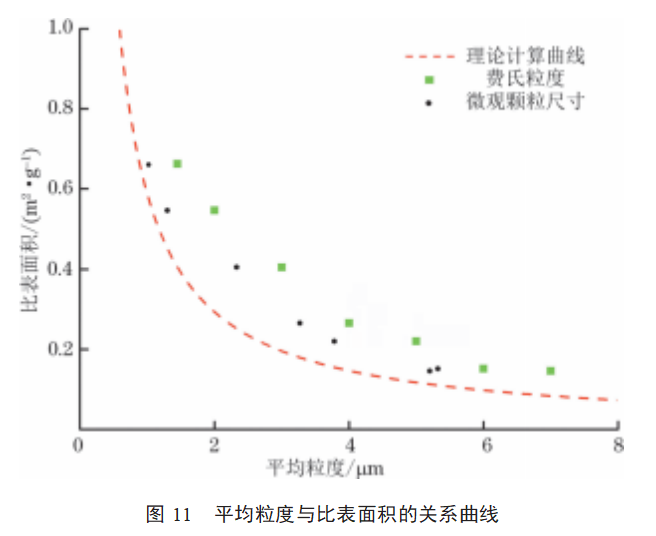
The theoretical calculation curves for the specific surface area B and particle size d of molybdenum powder were plotted based on the calculation results from Equation (1). Samples 8 and 9 exhibited severe agglomeration and clumping, which affected the measurement of specific surface area. Therefore, the measurement results of samples 8 and 9 were not used in the data analysis. The Fritsch particle size, microscopic particle size, and specific surface area of samples 1–7 were substituted into the theoretical calculation curve, with the results shown in Figure 11. As shown in Figure 11: The morphology of molybdenum powder particles varies, and the particle size is uneven. Therefore, the Fritsch particle size and microscopic particle size of the samples do not fully align with the theoretical calculation curve for the actual specific surface area results, but the overall trend generally follows the theoretical calculation trend; The relationship between the microscopic particle size of molybdenum powder and the measured specific surface area is closer to the theoretical calculation curve. When the particle morphology of molybdenum powder is spherical, the more uniform the particle size and the lower the agglomeration degree, the closer the measured specific surface area results are to the theoretical calculation results.
4 Conclusions
(1) The average particle size of molybdenum powder is inversely proportional to the specific surface area. The relationship between the Fehrert number and microscopic particle size and the actual specific surface area generally aligns with the trend of theoretical calculations.
(2) The agglomeration degree and abnormal morphology of molybdenum powder affect the measurement results of the Fehrert particle size and particle size distribution.
(3) The relationship between the microscopic particle size and the measured specific surface area is closer to the theoretical calculation curve than the relationship between the Fehrert particle size and the measured specific surface area.
(4) The closer the particle size distribution curve of molybdenum powder is to a single peak of a normal distribution, the more the relationship between particle size and specific surface area conforms to the theoretical calculation规律.
(5) When the particle morphology of molybdenum powder is spherical, the more uniform the particle size and the lower the agglomeration degree, the closer the measured specific surface area results are to the theoretical calculation results.
Reference: Chinese Library Classification: TB31; TG115.2 Document Type: A Article Number: 1001-4012(2024)07-0018-05 Relationship between molybdenum powder particle size and specific surface area
Xingchen Technology's spherical molybdenum powder is produced using radio frequency plasma spheroidization technology, featuring high purity (>99.95%), low oxygen content (<300 ppm), and excellent sphericity, making it suitable for applications such as 3D printing and additive manufacturing. The powder exhibits good flowability and uniform particle size distribution, making it suitable for precision forming processes such as laser selective melting (SLM) and electron beam melting (EBM). It performs exceptionally well in aerospace, medical devices, and high-temperature structural component manufacturing, meeting high-precision and high-strength requirements while supporting customized packaging and specifications. For more details, please contact our professional staff member Cathie Zheng at +86 13318326187.


